Abstract
Severe malaria in pregnancy is a large contributor to maternal morbidity and mortality. Intravenous quinine has traditionally been the treatment drug of choice for severe malaria in pregnancy. However, recent randomized clinical trials (RCTs) indicate that intravenous artesunate is more efficacious for treating severe malaria, resulting in changes to the World Health Organization (WHO) treatment guidelines. Artemisinins, including artesunate, are embryo-lethal in animal studies and there is limited experience with their use in the first trimester. This review summarizes the current literature supporting 2010 WHO treatment guidelines for severe malaria in pregnancy and the efficacy, pharmacokinetics, and adverse event data for currently used antimalarials available for severe malaria in pregnancy. We identified ten studies on the treatment of severe malaria in pregnancy that reported clinical outcomes. In two studies comparing intravenous quinine with intravenous artesunate, intravenous artesunate was more efficacious and safe for use in pregnant women. No studies detected an increased risk of miscarriage, stillbirth, or congenital anomalies associated with first trimester exposure to artesunate. Although the WHO recommends using either quinine or artesunate for the treatment of severe malaria in first trimester pregnancies, our findings suggest that artesunate should be the preferred treatment option for severe malaria in all trimesters.
Similar content being viewed by others
Intravenous artesunate is the most efficacious treatment for severe malaria, but data are limited on its use in pregnancy |
No associations have been reported between artemisinin use in humans and increased risk of miscarriage, stillbirth, or congenital anomalies |
The 2010 WHO guidelines recommend using either quinine or artesunate for the treatment of severe malaria in first trimester pregnancies |
Current data suggest that WHO treatment guidelines should be changed to recommend artesunate as the preferred treatment option for severe malaria in pregnancy during all three trimesters |
1 Introduction
An estimated 54.7 million pregnancies occurred in areas of stable Plasmodium falciparum malaria transmission, and an additional 70.5 million pregnancies occurred in areas of low transmission or areas with only Plasmodium vivax malaria in 2007 [1, 2]. Malaria in pregnancy is associated with higher rates of parasitemia, severe anemia, hypoglycemia, and acute pulmonary edema than malaria in non-pregnant women [3]. Malaria in pregnancy is a major contributor to morbidity and mortality, resulting in an estimated 100,000 neonatal deaths and 10,000 maternal deaths annually [4, 5]. Severe malaria is defined as acute malaria with major signs of organ dysfunction and/or high levels of parasitemia [3]. Pregnant women living in areas of unstable transmission are at higher risk of severe malaria and corresponding mortality [6]. Pregnant women in such areas have a three-times higher risk of severe malaria than do non-pregnant women [7]. Despite the substantial morbidity and mortality associated with severe malaria in pregnancy, pregnant women are often excluded from randomized trials of treatment drugs due to ethical concerns about the safety of these drugs for pregnant women and their fetuses [8].
The management of severe malaria in pregnancy needs to weigh the risks and benefits of treating malaria and the potential for adverse events for the pregnancy and fetus. Early treatment is critical because fever and illness of the mother are major risk factors for adverse pregnancy outcomes beyond the effects of the malaria parasite itself. The World Health Organization (WHO) guidelines stress prioritizing the life of the mother when treating severe malaria [9]. In choosing an appropriate therapy, one must weigh the known risks to the life of the mother compared with the uncertain risks of the treatment on the fetus.
This review summarizes the current literature available on the treatment of severe malaria in pregnancy. We review the data used to support 2010 WHO treatment guidelines for severe malaria in pregnancy and assess whether the current data support these guidelines.
2 Literature Review Methodology
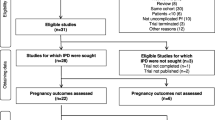
In order to identify all studies of the treatment of severe malaria in pregnancy, we conducted a review informed by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [10]. To identify all articles on severe malaria in pregnancy, we searched MEDLINE and Embase with the terms (‘severe malaria’ OR ‘complicated malaria’ OR ‘falciparum malaria’ OR ‘cerebral malaria’) AND ‘pregnancy’ AND (‘treatment’ OR ‘artemisinin’ OR ‘artesunate’ OR ‘artemether’ OR ‘quinine’) published before August 2014. We limited our search to English language articles. We also reviewed the references of key publications to ensure that all relevant literature was included in the review. We reviewed all of the literature that included the treatment of severe malaria in pregnancy. We included studies that reported the specific drug treatments pregnant women received and reported outcomes by drug regimen. Outcomes of interest included measures of efficacy in pregnancy, safety, and pharmacokinetics. The full results of our search are outlined in Fig. 1.
Data are scarce on treatment of severe malaria in pregnancy, so this review presents data on the drugs currently recommended for the treatment of severe malaria in pregnancy from studies of the treatment of uncomplicated malaria in pregnancy and the treatment of severe malaria in adults. This information was included in order to fully describe the safety and efficacy of the currently used therapies for the treatment of severe malaria in pregnancy. We used data from both the systematic review of the treatment of severe malaria in pregnancy and the key studies of uncomplicated malaria in pregnancy and severe malaria in adults to inform our recommendations.
3 Severe Malaria
3.1 Definition
The WHO defines severe P. falciparum malaria as a patient with P. falciparum asexual parasitemia with evidence of organ dysfunction (Fig. 2). The WHO definition of severe malaria applies for all patients, including pregnant women. A case of uncomplicated malaria can rapidly progress to severe malaria due to either delayed treatment or treatment failure.
3.2 Epidemiology of Severe P. falciparum Malaria in Pregnancy
Pregnant women have a three-times higher risk of severe malaria than non-pregnant women [7, 11]. Before the introduction of malaria control programs in Western Thailand, 1 % (5/500) of pregnant women died of malaria in a year [12]. The clinical presentation of severe malaria varies by age and pregnancy status, with adults presenting with hyperparasitemia, jaundice, and renal insufficiency more often than children [13]. In a malaria epidemic in India, severe anemia and cerebral malaria were more common in pregnant women with severe malaria than in non-pregnant women with severe malaria [20 % (5/45) versus 4 % (10/243), and 76 % (34/45) versus 33 % (80/243); p value <0.05], respectively [14]. Pregnant women with malaria are also at increased risk of hypoglycemia, acute respiratory distress syndrome (ARDS) [15], and hyperparasitemia [3] than non-pregnant women. In a review of severe malaria in pregnancy in the Asia Pacific region (227 women in eight studies) [14, 16–22], the median mortality rate was 39 % but ranged from 8 to 100 % [6]. This wide range is related to the broad WHO definition of severe malaria: the lowest mortality was reported in pregnant women whose only sign of severity was hypoglycemia. By contrast, all women with renal failure died [6]. There are limited mortality data from sub-Saharan Africa, but the percentage of maternal deaths attributed to malaria may be very similar between low- and high-transmission areas [4, 23, 24]. Two studies conducted in Sudan noted no maternal deaths among the pregnant women with severe malaria [25, 26]. In a retrospective case review of all maternal deaths 1985–1999, severe malaria was responsible for up to one-third of all maternal deaths in Sudan, a low-transmission setting [27]. Severe malaria accounted for 10 % of all deaths in an autopsy study of maternal mortality in Mozambique [24], and, similarly, was attributed to 9 % of maternal deaths in Western Kenya (a high-transmission setting) [28]. In addition to risk of death for the mother, infants born to women with clinical malaria infections late in pregnancy are at higher risk of early neonatal death during infancy [29, 30]. Young maternal age is an independent risk factor for severe malaria, with older age providing some protection [4]. Obstetric help should be sought, as severe malaria usually precipitates premature labor [31]. Even when the mother survives, there remains an increased risk of miscarriage and stillbirth, prematurity, and low birth weight for the fetus [6, 7, 9, 29, 32–34].
3.3 Epidemiology of Severe P. vivax Malaria
P. vivax malaria is not as benign as previously thought; recent data suggest that there is an increasing burden of morbidity and mortality due to P. vivax malaria in pregnant women [11, 35–38]. In high-prevalence areas such as regions in India, Myanmar, Asia Pacific, and South America, there have been cases where the P. vivax infections behaved more like P. falciparum [39–41]. P. vivax infections may trigger immune responses that could lead to potentially lethal clinical episodes independently or synergistically with other conditions or co-infections [42]. This inflammatory potential, in combination with P. vivax-induced hemolysis, increased red cell fragility, dyserythropoiesis, and retention of erythrocytes within the spleen contributes to severe anemia [43–45]. Localized exacerbations in the lung microvasculature have been proposed to cause ARDS [46]. Jaundice, renal failure, severe thrombocytopenia, acidosis, and, rarely, coma have also been reported in association with P. vivax [39]. In pregnancy, P. vivax malaria was reported to be associated with moderate anemia, but severe anemia could develop in pregnancy due to P. vivax malaria [47–50]. However, severe anemia in pregnancy may develop gradually via recurrent P. vivax episodes, and may not be reported as severe malaria in many settings. The burden of P. vivax malaria, and its severe manifestations, has been complicated by the emergence of resistance to chloroquine [39, 43].
The epidemiology of severe P. vivax malaria in pregnancy is difficult to determine. In the search for this manuscript, only four P. vivax-related maternal deaths were reported in the published literature. A cohort of 4,300 women with P. vivax malaria on the Thai-Myanmar border included one P. vivax-related maternal death: a third gravid, moderate anemic (known Β-thalassemia trait) woman who was 36 weeks pregnant died from ARDS 7 days after commencement of treatment for P. vivax malaria (P. vivax-related malaria mortality 23/100,000 live births) [51]. In a cohort from Indonesia, 456 women were diagnosed with P. vivax malaria, ten of whom had severe P. vivax disease. Two of these women died; P. vivax-related maternal mortality 439/100,000 (2/456) [52]. In a description of malaria-infected placentas from Mandla district in India, the authors describe a woman with a fairly high P. vivax parasitemia, jaundice, and severe anemia who died before labor started [53].
4 Current Treatment Guidelines
4.1 World health organization treatment guidelines
In order to combat the burden of malaria in pregnancy, the WHO Malaria Policy Advisory Committee (MPAC) recommends a three-pronged approach, including the use of long-lasting insecticide-treated nets, intermittent preventative therapy (IPT) by which pregnant women receive a dose of sulphadoxine-pyrimethamine (SP) at each antenatal care visit after the first trimester, and effective case management [54]. For pregnant women who are infected with P. falciparum, the WHO recommends treatment with artemisinin-based combination therapies (ACTs) during the second and third trimesters [55]. Artemisinins are given in combination with other antimalarial drugs to prevent the development of drug resistance. The WHO does not recommend the use of ACTs in the first trimester unless there is no alternative and the life of the woman is in danger [5].
Table 1 summarizes the current WHO 2010 treatment guidelines for severe P. falciparum malaria in pregnancy. In the treatment of severe malaria in pregnancy, saving the life of the mother is the primary objective. Intravenous artesunate has been shown to significantly reduce the risk of death from severe malaria compared with intravenous quinine in adults and children with severe malaria [20, 56]. In a study of 1,461 adults, artesunate was associated with a 34.7 % [95 % confidence interval (CI) 18.5–47.6] reduction in mortality compared with quinine for the treatment of severe malaria [20]. A similar study in 5,424 children found a 22.5 % (95 % CI 8.1–36.9) reduction in mortality associated with treatment with artesunate compared with quinine. Therefore, the WHO guidelines recommend intravenous artesunate in preference over quinine in the second and third trimester for severe malaria. Quinine is associated with recurrent hypoglycemia. However, in the first trimester, the risk of hypoglycemia is lower and the uncertainties over the safety of artemisinin derivatives is greater. The WHO recommends using either quinine or artesunate for the treatment of severe malaria in first-trimester pregnancies, until more evidence becomes available [57].
Although the WHO recommends artesunate as the preferred treatment for severe malaria in pregnancy, the USA, through the Centers for Disease Control and Prevention (CDC), recommends the use of quinidine (Figs. 2, 3). Quinidine is associated with arrhythmias, and a recent case series of patients hospitalized in the USA with malaria noted that four of six patients receiving quinidine experienced QT prolongation and two of six patients experienced Torsades de Pointes, a life-threatening arrhythmia associated with QT prolongation [58].
The evidence used to support the WHO treatment guidelines was based, in part, on a Cochrane review conducted in 2007 [59], which was updated in 2012 [60]. Treatment with artesunate significantly reduced the risk of death both in adults [relative risk (RR) 0.61, 95 % CI 0.50–0.75; 1,664 participants, five trials] and children (RR 0.76, 95 % CI 0.65–0.90; 5,765 participants, four trials) [60]. Only one study in the systematic review, the South East Asian Quinine Artesunate Malarial Trial (SEAQUAMAT), enrolled pregnant women and presented results stratified by pregnancy status [20]. In this large trial of 1,461 participants, 23 women in the artesunate arm and 26 in the quinine arm were pregnant at enrollment, but trimester of pregnancy was not reported. Mortality among pregnant women with severe malaria was 9 and 12 % [odds ratio (OR) 0.75; 95 % CI 0.09–6.41] for artesunate and quinine arms, respectively [20]. The WHO justified their recommendation for the use of artesunate instead of quinine despite limited data in pregnancy due to the large reduction in mortality associated with artesunate and the increased risk of hypoglycemia associated with the use of quinine [57]. Treatment must not be delayed; if only one of the drugs artesunate, artemether, or quinine is available, then it should be started immediately. We conducted an updated review of the literature using the same search terms as Dondorp et al. [56] (‘malaria’, ‘cerebral malaria’, ‘severe malaria’, ‘complicated malaria’, ‘malaria falciparum’, ‘quinine’, and ‘cinchona alkaloids’ and ‘artemisinin’ and ‘artesunate’), but no new clinical trials including pregnant women were identified.
4.1.1 World Health Organization Treatment Guidelines for Severe P. vivax Malaria
Severe malaria has been assumed to be associated with P. falciparum infection, but a recent review highlighted the substantial burden of severe malaria due to P. vivax infections [38]. While pregnant women were excluded, the results of meta-analysis showed a similar incidence of adult severe malaria due to P. vivax infections or P. falciparum mono-infection in adults [38]. In all trimesters of pregnancy, the WHO recommended treatment for uncomplicated P. vivax malaria is chloroquine, a total dose of 25 mg base/kg body weight in 3 days [57]. In an area with chloroquine-sensitive parasites, chemoprophylaxis with weekly chloroquine (500 mg phosphate or 300 mg base) prevented any recurrent P. vivax episodes in pregnant women [61]. However, reports of chloroquine-resistant parasites in pregnant women suggest that alternative treatment guidelines are needed to prevent repeated dosing of an ineffective drug [62]. Many countries in the Asia-Pacific region have changed the first-line treatment of P. vivax malaria to ACTs. Quinine and clindamycin remain the treatment of choice for P. vivax malaria in the first trimester [52].
For pregnant patients with severe P. vivax malaria, the WHO recommends the same treatments as for severe P. falciparum infections (Table 1) [57]. P. vivax has liver stages (hypnozoites) that cause recurrent blood-stage infections (relapses). Primaquine—the only drug effective against liver stages—is contraindicated in pregnant women because fetal red blood cells are susceptible to hemolysis [63]. Pregnant women should receive suppressive prophylaxis with chloroquine until delivery, at which point they can receive radical treatment with primaquine [61].
5 Summary of Drugs for Treatment of Severe Malaria
5.1 Artemisinins
The most efficacious drugs for treating severe malaria are artemisinin-based compounds. Artemisinins, derived from the plant Artemisia annua, have been used for centuries in China to treat fever. Currently, three artemisinin compounds are used to treat malaria: artesunate, artemether, and dihydroartemisinin.
5.1.1 Safety of Artemisinins in Pregnancy
Despite their proven efficacy, artemisinins are embryotoxic and teratogenic in animal studies [64]. Artesunate is toxic to rat and rabbit embryos, with fetal reabsorption in rats reported at low doses (28–223 mg/kg/day) given orally on days 9–14 of gestation [55]. High rates of congenital malformations, including bent and/or shortened long bones and treatment-related heart defects, were observed in rat litters [55]. Primate studies demonstrated similar embryo toxicity, with 55 and 100 % embryo lethality for 12 and 30 mg/kg/d oral doses, respectively, after 12 days of treatment [65]. In addition, observations of three live primate embryos from the 30 mg group noted reductions in blood cells in the vasculature, and the cardiac chambers were distended with thin walls [65]. The primary embryonic targets for artemisinins are the primitive erythroblasts proliferating during gestational periods of 10–14 days in the rat and 18–40 days in the monkey. In humans, this corresponds to a sensitive period from post-conception week 3 to approximately post-conception week 9 [65].
The findings from animal reproductive toxicology studies and the dearth of human studies led the WHO to not recommend the use of artemisinins in the first trimester of pregnancy for uncomplicated malaria. The WHO recommends that artesunate may be used to treat severe malaria in the first trimester because of the high rates of mortality, stillbirth, and miscarriage associated with severe malaria [57]. Observational data on the safety and efficacy of artemisinins in the first trimester of pregnancy are very limited. A recent systematic review identified eight studies of antimalarials from 1997 to 2013 that included first-trimester pregnancies and a total of 35 studies including pregnant women of any gestational age, but the vast majority of patients had uncomplicated malaria [66]. The systematic review identified 555 first-trimester exposures to artemisinins in clinical trials [66], and an additional 945 women with a single malaria episode in the first trimester were described along the Thai-Burma border, 92 of whom were exposed to an artemisinin [9]. Neither the clinical trials nor the large cohort study observed an increased risk of adverse pregnancy outcomes associated with exposures to artemisinins. Birth outcomes did not differ significantly for women exposed to artesunate compared with quinine and chloroquine during the first trimester, and artemisinins were not associated with miscarriage after controlling for maternal and malaria characteristics [9].
Studies in animals suggest that artesunate is a teratogen when given alone or in combination with mefloquine [67, 68]. The limited safety data in humans have not shown any increased risk of miscarriage, stillbirth, or congenital anomalies associated with exposure during the first trimester or later in pregnancy [9, 69–72]. Animal studies suggest that, similar to artesunate, artemether is embryotoxic in rat models [73, 74]. The limited safety data in humans have failed to find an association with artemether exposure and increased risk of stillbirth, miscarriage, or congenital anomalies [69, 75–77].
5.1.2 Artesunate
Artesunate is a water-soluble artemisinin derivative. The drug is available orally in combination with other antimalarials, or in rectal and parenteral formulations used for the treatment of severe malaria. Artesunate is associated with faster parasite clearance than quinine because the drug targets young circulating ring-stage parasites [78, 79]. Artesunate does not require a loading dose. For the treatment of severe malaria, the WHO recommends 2.4 mg/kg to be given immediately intravenously or by intramuscular injection, with repeat doses at 12 and 24 h. Continued injections can be given once daily as necessary.
A review of the literature identified three pharmacokinetic studies of artesunate in pregnant women. Pharmacology data suggest that pregnant women receiving oral artesunate may have accelerated clearance rates of the metabolite dihydroartemisinin compared with non-pregnant women [80, 81]. A single study of intravenous and oral artesunate noted no significant differences in the pharmacokinetics between pregnant women with malaria compared with the same women in a healthy state after administration of intravenous artesunate. The same study noted that pregnant women with malaria had significantly higher drug exposure after receiving oral artesunate than the same women when healthy and not pregnant [82].
Clinical data on the efficacy of artesunate in the treatment of severe malaria in pregnancy are limited [9, 20, 52]. Only the SEAQUAMAT trial reported data on pregnant women with severe malaria comparing artesunate with quinine in a clinical trial setting. A systematic review by Visser et al. [66] identified ten studies reporting outcomes of pregnant women treated with artesunate. Artesunate is associated with higher cure rates, lower gametocyte carriage, and lower treatment failure than quinine-based therapies for the treatment of uncomplicated malaria in pregnancy [69, 70, 72, 83, 84]. Our systematic review of the literature identified two additional studies presenting data on artesunate to treat severe malaria in pregnancy [9, 52]. Poespoprodjo et al. [52] found no statistically significant increased risk of poor maternal or fetal outcomes associated with artesunate compared with quinine, and noted than none of the ten women exposed to intravenous artesunate in the first trimester had a miscarriage. McGready et al. [9] noted that, although miscarriage rates were very high for women with severe malaria in the first trimester (58 %), there was no difference in the rates between pregnant women treated with artesunate or quinine.
Artesunate is generally associated with better tolerability than quinine [20, 83, 85]. The most important reported side effect of artesunate is anaphylaxis, with an estimated risk of approximately 1 in 3,000 patients. Additional common minor side effects associated with intravenous administration include dizziness, light-headedness, rash, and taste alterations. Nausea, vomiting, and diarrhea have been reported, but it is unclear whether these are associated with artesunate or are symptoms of severe malaria [86]. Cardiotoxicity has been observed after administration of high doses of artesunate [87]. Multiple studies in adults and children suggest that up to 25 % of patients treated with intravenous artesunate for severe malaria developed delayed hemolytic anemia 7–31 days after treatment, but the etiology is unknown [88–92].
5.1.3 Artemether
Artemether is a commonly used artemisinin for the treatment of uncomplicated malaria when combined with lumefantrine (marketed as Coartem®). According to the WHO, artemether can be used to treat severe malaria in pregnancy when artesunate is unavailable [57]. Artemether should be given at 3.2 mg/kg body weight intramuscularly at admission and then 1.6 mg/kg body weight per day as needed. The parenteral formulation is oil based; data suggest that it is poorly or erratically absorbed in this formulation [93].
A review of the literature identified four pharmacokinetic studies of artemether in pregnant women. All four studies demonstrated that pregnant women have lower concentrations of artemether and its metabolite dihydroartemisinin than non-pregnant controls [94–97]. These results suggest that further research is needed to identify the correct oral dose of artemether for pregnant women to decrease the likelihood of treatment failure and to prevent resistance. No studies were identified that examined the pharmacokinetics of artemether as an injection in pregnant women, but, because it is oil based it is hypothesized that absorption is erratic [93].
A systematic review of artemether for the treatment of severe malaria, conducted in 2001 using data from seven trials, noted that 14 % of those treated with artemether and 17 % of those treated with quinine died (OR 0.08, 95 % CI 0.62–1.02; p = 0.08), but none of the trials included pregnant women [98–102]. The authors concluded that artemether was equivalent to quinine for the treatment of severe malaria [103]. The systematic review noted that treatment with artemether was not associated with an increased number of adverse events compared with quinine, but artemether was associated with significantly faster parasite clearance, hazard ratio (HR) 0.62 (95 % CI 0.56–0.69). An updated systematic review of the literature by the authors failed to identify any studies published since 2001 that included results for pregnant women.
A systematic review of the treatment of malaria in pregnancy by Visser et al. identified 16 studies reporting pregnancy outcomes in women exposed to artemether–lumefantrine (AL) or artemether for treatment of uncomplicated malaria [66, 69, 72, 75, 76, 94, 104–109]. An additional systematic review of the use of artemether and AL to treat uncomplicated malaria in pregnancy identified 1,103 reports of AL use in pregnancy [110]. Broadly, the efficacy of artemether was non-inferior or superior to that of quinine. Specifically, one study comparing quinine with artemether combined with atovaquone–proguanil (A + AP) saw much higher day 63 cure rates in the A + AP group than in the quinine group [94.9 % (95 % CI 81.4–99.1) vs. 63.4 % (95 % CI 46.9–77.4) respectively] [109].
Intramuscular artemether is generally well tolerated, with very few side effects reported [93, 111–113]. Pain and swelling at the injection site is a common, mild side effect [113]. Cardiotoxicity has been observed rarely, resulting in arrhythmias such as ventricle tachycardia [87].
We identified two trials, both conducted in Vietnam, that directly compared artemether with artesunate for treating severe malaria in adults [114, 115]. Both trials excluded women pregnant in their first trimester and did not report results stratified by pregnancy status. One trial compared four different regimens with no statistically significant difference in mortality across the treatments. The mortality rates were 11.1 % (5/45) for intramuscular artemether, 17.6 % (9/51) for artemisinin suppositories, 10.2 % (6/49) for intramuscular artesunate, and 17.6 % (5/30) for intravenous artesunate [114]. A second double-blind trial conducted between 1996 and 2003 compared intramuscular artesunate with intramuscular artemether for the treatment of severe malaria. The trial reported 7 % (13/186) mortality in the artesunate group and 13 % (24/184) mortality in the artemether group; however, the results were not statistically significant (p = 0.052). The authors concluded that intramuscular artemether may not be the best artemisinin therapy for treating severe malaria because the drug releases slowly and erratically compared with water-soluble artesunate [115].
5.2 Quinine
Quinine was first discovered as a treatment for malaria in the 17th century [85]. Quinine is available as oral or intravenous preparations and reaches peak concentrations 1–3 h after administration [116]. Quinine attacks intra-erythrocytic malaria parasites, and it has gametocytocidal properties against P. vivax and Plasmodium malariae but not P. falciparum [85]. The pharmacokinetic properties of quinine vary by age of the patient and malaria status.
Unlike artemisinin-based therapies, quinine is not considered embryotoxic or teratogenic in studies of rats [117], rabbits [55], dogs [55], or macaque monkeys [118]. The primary safety concern for quinine in pregnancy is the potential for auditory nerve damage [55]. Quinine exposures during the first trimester were not associated with an increased risk of congenital malformation in refugees along the Thai-Burma border [9, 70]. One study reported an increased risk of miscarriage and stillbirth associated with first-trimester exposure to quinine compared with AL [119]. Despite previous concerns, quinine at treatment doses does not induce abortion or labor [18].
A literature review identified six studies of the pharmacokinetics of quinine for treating uncomplicated malaria in pregnancy [95, 120–123]. The results of these studies varied in their findings, with some studies suggesting that that was no difference in the metabolism of quinine between pregnant and non-pregnant women [122], and other studies suggesting that pregnant women metabolize quinine faster than non-pregnant women, requiring further research into the proper dosing for pregnancy.
Despite quinine being used for hundreds of years for the treatment of severe malaria in pregnancy, the systematic review identified only ten relevant studies [9, 14, 16, 19, 20, 25, 26, 52, 124, 125] (Tables 2, 3). Of concern, quinine has been associated with a higher risk of hypoglycemia, which can be fatal, than treatment with artemisinins [126]. A recent systematic review identified ten studies conducted between 1997 and 2013 on the efficacy of quinine compared with artemisinin-based therapies for the treatment of uncomplicated malaria in pregnancy [66]. McGready et al. [70] reported statistically significantly lower 63-day cure rates for quinine when compared with mefloquine + artesunate (67 vs. 98.2 %; p = 0.0001) for the treatment of uncomplicated malaria [70]. Quinine + clindamycin had similar cure rates to those of artesunate, but significantly higher rates of side effects [83]. Additional studies noted very high mortality rates among pregnant women with severe malaria: from 0 to 80 % for those treated with intravenous quinine [16, 25].
Intravenous quinine is less well tolerated than intravenous artesunate [83]. Mild to moderate cinchonism, including tinnitus, headache, blurred vision, altered auditory acuity, nausea, and diarrhea often arise after 3 days of treatment [87]. Poor tolerability of oral quinine has been linked to lower adherence and subsequent treatment failures in patients [127–129]. A study of the compliance with artesunate compared with quinine + tetracycline found that non-pregnant patients who were taking artemisinins were more likely to be compliant with treatment [adjusted RR (aRR) 1.39, 95 % CI 1.15–1.68], and the reasons cited for non-compliance were adverse drug reactions and forgetting to take drugs [127]. Tinnitus is the most commonly reported side effect in pregnant women [85]. Pregnant women are also at risk for a rare triad of complications: massive hemolysis, hemoglobinemia, and hemoglobinuria [130]. Additional side effects include muscle necrosis and sterile abscesses due to intramuscular injections. Dose-related adverse events include cardiovascular symptoms such as transient ventricular tachycardia, and gastrointestinal and central nervous system disorders resulting from excessive infusions [87].
5.3 Partner Drugs
After initial treatment for severe malaria with intravenous quinine or artesunate, patients should receive oral regimens. The WHO recommends either quinine + clindamycin for 7 days or an oral ACT for the treatment of pregnant women [57], but the safety of these partner drugs should also be considered [131]. Quinine + clindamycin is recommended by the WHO for the treatment of malaria in the first trimester. Clindamycin, a common partner drug, has been associated with abdominal side effects, including nausea, vomiting, and diarrhea [132, 133]. However, a more recent RCT comparing quinine + clindamycin and artesunate for the treatment of uncomplicated malaria in pregnancy did not report abdominal side effects, but women in the trial taking quinine + clindamycin did experience significantly higher rates of tinnitus than did the artesunate group [83]. Because of relatively high rates of side effects and the high cost per treatment (US$18.50) [83], quinine + clindamycin may result in poor adherence in resource-poor settings.
6 Special Considerations
6.1 HIV and Severe Malaria in Pregnancy
Pregnant women have the greatest burden of the morbidity and mortality associated with co-infection with HIV and malaria [134]. HIV infection is associated with a greater risk of severe malaria [135, 136]. Despite this large burden, very little information exists about interactions between ACTs and antiretroviral therapy (ART) and the optimal treatment options for severe malaria in pregnant women with HIV. One study of the pharmacokinetics of quinine in HIV-positive pregnant women noted that quinine concentrations may be lower in women receiving nevirapine-based ART [123]. The WHO recommends that HIV-infected patients receiving zidovudine or efavirenz should avoid amodiaquine-containing ACT regimens because of reports of hepatotoxicity [57]. A review of the concurrent management of HIV in pregnancy recommends that women receiving highly active antiretroviral therapy (HAART) regimens including protease inhibitors (PIs) and/or non-nucleotide reverse transcriptase inhibitors (NNRTIs) receive quinine when cardiac monitoring is available. Parenteral artesunate or artemether should be used when quinine and cardiac monitoring are not available because of an increased risk of cardiotoxicity associated with quinine [137, 138]. Women receiving no PIs or NNRTIs may be treated with either parenteral quinine or artesunate [139]. Current clinical data suggest that artemether when co-administered with PIs or NNRTIs may result in a reduction in the active metabolite of artemether, but the clinical importance is unclear [137].
6.2 Rectal Administration of Antimalarial Drugs for Severe Malaria in the Community
The WHO recommends rectal administration of artesunate as a pre-referral treatment for severe malaria [57]. There are currently no data comparing rectal administration with intravenous and intramuscular injections of artemisinin derivatives; therefore, the WHO guidance states that suppositories only be used for pre-referral treatment. Furthermore, there are no studies of rectal administration of artesunate to treat malaria in pregnancy. According to the WHO, if referral is impossible, patients may be treated with artesunate suppositories until able to receive oral medication. A systematic review of randomized clinical trials of pre-referral rectal administration of artesunate identified only one study. The study found a protective effect in young children who received rectal artesunate compared with placebo (RR 0.74, 95 % CI 0.59–0.93), but older children and adults were at greater risk of death than the placebo group (RR 2.21, 95 % CI 1.18–4.15) [140]. Despite these contradictory findings, it is critical to treat malaria early, especially in pregnancy.
6.3 Drug Quality
Poor-quality antimalarials have important health consequences, including the potential for treatment failure, development of antimicrobial resistance, and serious adverse drug reactions. Treatment with counterfeit oral artesunate has led to deaths due to severe malaria [141]. Reports from surveys conducted in sub-Saharan Africa (SSA) samples and south-east Asia indicate that orally administered antimalarial drugs are commonly of poor quality [142]. Nayyar et al. [143] reviewed published and unpublished studies and found that 35 % (497/1,437) of samples from south-east Asia failed chemical testing. Using the same criteria, this analysis also reported that 35 % (796/2,297) of samples from SSA failed chemical analysis. Substandard drugs fail to meet the specifications set forth by a regulatory agency and as specified in a standard pharmacopeia or the manufacturer’s dossier. Most surveys of drug quality emphasize sampling from licensed pharmacies and dispensaries, not hospitals or even the informal, unregulated markets. WHO pre-qualification process that includes dossier reviews, site inspections, and recognition of approval by stringent regulatory agencies are the methods used by donors for assuring the quality of medicines, including antimalarials [144]. One manufacturer of injectable artesunate, Guilin Pharmaceuticals, achieved WHO pre-qualification status in 2010 [145]. A multitude of methods are used to assess the quality of medicines and can be implemented at the health facilities and ports of entry [146]. Ensuring the quality and safety of antimalarials is critical in the treatment of severe malaria.
7 Discussion and Conclusions
Severe malaria (P. falciparum as well as P. vivax malaria) in pregnancy presents an immediate danger to the life of the mother and fetus, and therefore effective treatment must be delivered immediately. Despite the importance of malaria in pregnancy, we identified only ten studies that presented treatment outcomes of pregnant women with severe malaria, demonstrating a paucity of data. Multiple studies have shown that artemisinin derivatives, specifically artesunate, are more effective at treating severe malaria than quinine [9, 20, 52, 56]. Others have reported on the economic advantages of intravenous artesunate over intravenous quinine [147, 148]. Despite concerns about the signal from animal studies of the embryo-lethality and teratogenicity of artemisinins, no studies have shown an association between artemisinin exposures during pregnancy and increased risk of miscarriage, stillbirth, or congenital anomalies in humans [9, 75, 76]. Safety data suggest a need for strong pharmacovigilance, especially regarding the reported delayed hemolysis after treatment with intravenous artesunate. As the use of intravenous artesunate expands, the potential to detect rare but severe adverse events will increase with the proper surveillance. Additionally, pharmacovigilance systems are necessary to aid in combatting the threat of falsified and substandard antimalarials [149].
Given the high morbidity and mortality associated with severe malaria in pregnancy, additional research is needed on the pharmacokinetics of intravenous and intramuscular artemisinin derivatives in pregnancy. Multiple studies have suggested that pregnancy and malaria infection alter the metabolism of artemisinins, and therefore the ideal therapeutic dosages in pregnancy are unclear. Furthermore, additional research is needed on the efficacy of pre-referral use of rectal artesunate in pregnant women with severe malaria.
The current evidence suggests that intravenous artesunate should be used in preference to intravenous quinine for the treatment of severe malaria in first-trimester pregnancies. We recommend that WHO guidelines consider artesunate as the preferred treatment for severe malaria in pregnancy during all three trimesters in order to ensure that pregnant women receive the most efficacious treatment. We also recommend that the US Food and Drug Administration (FDA) approve artesunate for the treatment of severe malaria, therefore allowing the CDC to update its treatment guidelines to recommend the most efficacious treatment. Recommendations for the treatment of severe malaria in pregnancy must balance efficacy with prevention of serious adverse advents by incorporating new evidence as it becomes available.
References
Dellicour S, Hall S, Chandramohan D, Greenwood B. The safety of artemisinins during pregnancy : a pressing question. Malar J. 2007;10:1–10. doi:10.1186/1475-2875-6-15.
Dellicour S, Tatem AJ, Guerra CA, et al. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. doi:10.1371/journal.pmed.1000221.
White NJ, Pukrittayakamee S, Hien TT, et al. Malaria. Lancet. 2014;383:723–35. doi:10.1016/S0140-6736(13)60024-0.
Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi:10.1016/S1473-3099(07)70021-X.
World Health Organization (2012) World Malaria Report 2012.
Rijken MJ, McGready R, Boel ME, et al. Malaria in pregnancy in the Asia–Pacific region. Lancet Infect Dis. 2012;12:75–88. doi:10.1016/S1473-3099(11)70315-2.
Luxemburger C, Ricci F, Nosten F, et al. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62.
White NJ, McGready RM, Nosten FH. New medicines for tropical diseases in pregnancy: catch-22. PLoS Med. 2008;5:e133. doi:10.1371/journal.pmed.0050133.
McGready R, Lee SJ, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy : a population-based study. Lancet Infect Dis. 2012;. doi:10.1016/S1473-3099(11)70339-5.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;. doi:10.1136/bmj.b2700.
Sholapurkar SL, Gupta AN, Mahajan RC. Clinical course of malaria study from India in pregnancy—a prospective controlled study from India. Trans R Soc Trop Med Hyg. 1988;82:376–9.
Nosten F, ter Kuile F, Maelankirri L, et al. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg. 1991;85:424–9.
Dondorp AM, Lee SJ, Faiz MA, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–7. doi:10.1086/589287.
Kochar D, Thanvi I, Joshi A, et al. Falciparum malaria in pregnancy. Indian J Malariol. 1998;35:123–30.
Taylor WRJ, Hanson J, Turner GDH, et al. Respiratory manifestations of malaria. Chest. 2012;142:492–505. doi:10.1378/chest.11-2655.
Singh N, Shukla M, Valecha N. Malaria parasite density in pregnant women of district Jabulpur, Madhya Pradesh. Indian J Malariol. 1996;33:41–7.
Lalloo DG, Trevett AJ, Paul M, et al. Severe and complicated falciparum malaria in Melanesian adults in Papua New Guinea. Am J Trop Med Hyg. 1996;55:119–24.
Looareesuwan S, White N, Karbwang J, et al. Quinine and servere falciparum malaria in late pregnancy. Lancet. 1985;326(8445):4–6.
Kochar D, Shubhakaran Kumawat B, et al. Cerebral malaria in Indian adults: a prospective study of 441 patients from Bikaner, north-west India. J Assoc Physicians India. 2002;50:234–41.
Dondorp A, Nosten F, Stepniewska K, et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi:10.1016/S0140-6736(05)67176-0.
Hasan A, Parvez A, Shaheen, Shah A. Pregnancy in patients with malaria. Indian J Acad Clin Med. 2006;7:25–9.
Arya TV, Prasad RN, Virk KJ. Cerebral malaria in pregnancy. J Assoc Physicians India. 1989;37:592–3.
Barnett S, Nair N, Tripathy P, et al. A prospective key informant surveillance system to measure maternal mortality—findings from indigenous populations in Jharkhand and Orissa, India. BMC Pregnancy Childbirth. 2008;8:6. doi:10.1186/1471-2393-8-6.
Menéndez C, Romagosa C, Ismail MR, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. doi:10.1371/journal.pmed.0050044.
Adam I, Mighani O, Saed O, et al. (2004) Quinine therapy in severe Plasmodium falciparum malaria during pregnancy in Sudan. East Mediterr Heal J. 10:159–66.
Elbadawi NEE, Mohamed MI, Dawod OY, et al. Effect of quinine therapy on plasma glucose and plasma insulin levels in pregnant women infected with Plasmodium falciparum malaria in Gezira state. East Mediterr Health J. 2011;17:697–700.
Dafallah SE, El-Agib FH, Bushra GO. Maternal mortality in a teaching hospital in Sudan. Saudi Med J. 2003;24:369–72.
Desai M, Phillips-Howard PA, Odhiambo FO, et al. An analysis of pregnancy-related mortality in the KEMRI/CDC health and demographic surveillance system in western Kenya. PLoS One. 2013;8:e68733. doi:10.1371/journal.pone.0068733.
Bardají A, Sigauque B, Sanz S, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis. 2011;203:691–9. doi:10.1093/infdis/jiq049.
Luxemburger C, McGready R, Kham A, et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am J Epidemiol. 2001;154:459–65.
World Health Organization. Management of severe malaria: a practical handbook. 3rd ed. Geneva: Switzerland; 2012.
Menon R. Pregnancy and malaria. Med J Malaya. 1972;27:115–9.
Endeshaw Y. Malaria in pregnancy: clinical features and outcome of treatment. Ethiop Med J. 1991;29:103–8.
Nair LS, Nair AS. Effects of malaria infection on pregnancy. Indian J Malariol. 1993;30:207–14.
Kochar DK, Saxena V, Singh N, et al. (2005) Plasmodium vivax. Emerg. Infect. Dis. 11:132–4.
Nayak KC, Khatri MP, Gupta BK, et al. Spectrum of vivax malaria in pregnancy and its outcome: a hospital-based study. J Vector Borne Dis. 2009;46:299–302.
Price RN, Tjitra E, Guerra CA, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87.
Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is plasmodium vivax malaria a severe malaria? a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8:e3071. doi:10.1371/journal.pntd.0003071.
Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–5. doi:10.1097/QCO.0b013e32832f14c1.
Battle KE, Gething PW, Elyazar IRF, et al. (2012) Chapter one—the global public health significance of plasmodium vivax. In: Hay SI (ed) RP and JKBBT-A in P. Adv Parasitol. London: Academic Press, pp. 1–111.
Lacerda MVG, Mourão MPG, Alexandre MA, et al. Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar J. 2012;11:12. doi:10.1186/1475-2875-11-12.
Bassat Q, Alonso PL. Defying malaria: Fathoming severe Plasmodium vivax disease. Nat Med. 2011;17:48–9. doi:10.1038/nm0111-48.
Tjitra E, Hasugian AR, Siswantoro H, et al. Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria : a multi-centre study in Indonesia. Malar J. 2012;11:1–14.
Genton B, D’Acremont V, Rare L, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:e127. doi:10.1371/journal.pmed.0050127.
Anstey NM, Handojo T, Pain MCF, et al. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis. 2007;195:589–96. doi:10.1086/510756.
Valecha N, Pinto RGW, Turner GDH, et al. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–62. doi:10.4269/ajtmh.2009.09-0348.
Nosten F, McGready R, Simpson JA, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–9.
Poespoprodjo JR, Fobia W, Kenangalem E, et al. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374–81. doi:10.1086/586743.
Singh N, Shukla MM, Sharma VP. Epidemiology of malaria in pregnancy in central India. Bull World Health Organ. 1999;77:567–72.
Rodriguez-morales AJ, Sanchez E, Vargas M, et al. Short report: pregnancy outcomes associated with Plasmodium vivax malaria in Northeastern Venezuela. Am J Trop Med Hyg. 2006;74:755–7.
McGready R, Wongsaen K, Chu CS, et al. Uncomplicated Plasmodium vivax malaria in pregnancy associated with mortality from acute respiratory distress syndrome. Malar J. 2014;13:191. doi:10.1186/1475-2875-13-191.
Poespoprodjo JR, Fobia W, Kenangalem E, et al. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS One. 2014;9:e84976. doi:10.1371/journal.pone.0084976.
Singh N, Saxena A, Shrivastava R. Placental Plasmodium vivax infection and congenital malaria in central India. Ann Trop Med Parasitol. 2003;97:875–8. doi:10.1179/000349803225002688.
WHO Malaria Policy Advisory Committee and Secretariate. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar J. 2012;11:424. doi:10.1186/1475-2875-11-424.
Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1–15.
Dondorp AM, Fanello CI, Hendriksen ICE, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57. doi:10.1016/S0140-6736(10)61924-1.
World Health Organization. Guidelines for the treatment of malaria. 2nd ed. Geneva: Switzerland; 2010.
Wroblewski HA, Kovacs RJ, Kingery JR, et al. High risk of QT interval prolongation and torsades de pointes associated with intravenous quinidine used for treatment of resistant malaria or babesiosis. Antimicrob Agents Chemother. 2012;56:4495–9. doi:10.1128/AAC.06396-11.
Jones KL, Donegan S, Laloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev. 2007;(4). doi:10.1002/14651858.CD005967.pub2.
Sinclair D, Donegan S, Isba R, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev. 2012;(6). doi:10.1002/14651858.CD005967.pub4.
Villegas L, McGready R, Htway M, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop Med Int Health. 2007;12:209–18. doi:10.1111/j.1365-3156.2006.01778.x.
Rijken MJ, Boel ME, Russell B, et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J. 2011;10:113. doi:10.1186/1475-2875-10-113.
Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–45. doi:10.1086/424663.
Li Q, Weina PJ. Severe embryotoxicity of artemisinin derivatives in experimental animals, but possibly safe in pregnant women. Molecules. 2010;15:40–57. doi:10.3390/molecules15010040.
Clark RL. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol. 2009;28:285–96. doi:10.1016/j.reprotox.2009.05.002.
Visser BJ, van Vugt M, Grobusch MP. Malaria: an update on current chemotherapy. Expert Opin Pharmacother. 2014;. doi:10.1517/14656566.2014.944499.
Boareto AC, Müller JC, de Araujo SL, et al. Study on the developmental toxicity of combined artesunate and mefloquine antimalarial drugs on rats. Reprod Toxicol. 2012;34:658–64. doi:10.1016/j.reprotox.2012.10.004.
Clark RL, Lerman SA, Cox EM, Gristwood WE, White TEK. Developmental toxicity of artesunate and an artesunate combination in the rat and rabbit. Birth Defects Res B Dev Reprod Toxicol. 2004;71:380–94. doi:10.1002/bdrb.20027.
McGready R, Cho T, Keo NK, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis. 2001;33:2009–16. doi:10.1086/324349.
McGready R, Brockman A, Cho T, et al. Randomized comparison of mefloquine-artesunate versus quinine in the treatment of multidrug-resistant falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg. 2000;94:689–93.
McGready R, Nosten F. The Thai-Burmese border: drug studies of Plasmodium falciparum in pregnancy. Ann Trop Med Parasitol. 1999;93(Suppl 1):S19–23.
Mcgready R, Tan SO, Ashley EA, et al. A randomised controlled trial of artemether–lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;. doi:10.1371/journal.pmed.0050253.
Clark RL, Lerman SA, Cox EM, et al. Developmental toxicity of artesunate in the rat: comparison to other artemisinins, comparison of embryotoxicity and kinetics by oral and intravenous routes, and relationship to maternal reticulocyte count. Birth Defects Res B Dev Reprod Toxicol. 2008;83:397–406. doi:10.1002/bdrb.20165.
El-Dakdoky MH. Evaluation of the developmental toxicity of artemether during different phases of rat pregnancy. Food Chem Toxicol. 2009;47:1437–41. doi:10.1016/j.fct.2009.03.027.
Rulisa S, Kaligirwa N, Agaba S, et al. Pharmacovigilance of artemether–lumefantrine in pregnant women followed until delivery in Rwanda. Malar J. 2012;11:225. doi:10.1186/1475-2875-11-225.
Piola P, Nabasumba C, Turyakira E, et al. Efficacy and safety of artemether–lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis. 2010;10:762–9. doi:10.1016/S1473-3099(10)70202-4.
Manyando C, Mkandawire R, Puma L, et al. Safety of artemether–lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar J. 2010;9:249. doi:10.1186/1475-2875-9-249.
White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4. doi:10.1126/science.1155165.
Ter Kuile FO, White NJ, Holloway P, et al. Plasmodium falciparum. in vitro studies of the pharmacodynamic propoerties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:85–95.
Morris CA, Onyamboko MA, Capparelli E, et al. Population pharmacokinetics of artesunate and dihydroartemisinin in pregnant and non-pregnant women with malaria. Malar J. 2011;10:114. doi:10.1186/1475-2875-10-114.
McGready R, Stepniewska K, Ward SA, et al. Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62:367–71. doi:10.1007/s00228-006-0118-y.
McGready R, Phyo AP, Rijken MJ, et al. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: absorption, bioavailability, disposition and disease effects. Br J Clin Pharmacol. 2012;73:467–77. doi:10.1111/j.1365-2125.2011.04103.x.
Mcgready R, Cho T, Villegas L, et al. Randomized comparison of quinine-clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg. 2001;95:651–6.
Mutabingwa TK, Muze K, Ord R, et al. Randomized trial of artesunate + amodiaquine, sulfadoxine-pyrimethamine + amodiaquine, chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania. PLoS One. 2009;4:e5138. doi:10.1371/journal.pone.0005138.
Achan J, Talisuna AO, Erhart A, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi:10.1186/1475-2875-10-144.
Guilin Pharma (2012) Artesun® Artesunate for Injection (Package Insert).
World Health Organization. WHO model prescribing information: drugs used in parasitic diseases. 2nd ed. Geneva: Switzerland; 1995.
Zoller T, Junghanss T, Kapaun A, et al. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17:771–7. doi:10.3201/eid1705.101229.
Centers for Disease Control and Prevention. Published reports of delayed hemolytic anemia after treatment with artesunate for severe malaria worldwide. MMWR Morb Mortal Wkly Rep. 2013;62:1–4.
Kreeftmeijer-Vegter AR, van Genderen PJ, Visser LG, et al. Treatment outcome of intravenous artesunate in patients with severe malaria in the Netherlands and Belgium. Malar J. 2012;11:102. doi:10.1186/1475-2875-11-102.
Rolling T, Spahlinger D, Issifou S, et al. Extended haematological follow-up after parenteral artesunate in African children with severe malaria. Malar J. 2012;11:P83. doi:10.1186/1475-2875-11-S1-P83.
Jarvis JN, Coltart CEM, Pule M, et al. Artemisinin therapy and severe delayed haemolysis. Lancet. 2013;382:180. doi:10.1016/S0140-6736(13)60812-0.
Hien T, Day NP, Nguyen HP, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi:10.1056/NEJM199607113350202.
McGready R, Stepniewska K, Lindegardh N, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62:1021–31. doi:10.1007/s00228-006-0199-7.
Tarning J, Kloprogge F, Dhorda M, et al. Pharmacokinetic properties of artemether, dihydroartemisinin, lumefantrine, and quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. Antimicrob Agents Chemother. 2013;57:5096–103. doi:10.1128/AAC.00683-13.
Tarning J, Kloprogge F, Piola P, et al. Population pharmacokinetics of Artemether and dihydroartemisinin in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. Malar J. 2012;11:293. doi:10.1186/1475-2875-11-293.
Mosha D, Guidi M, Mwingira F, et al. Population pharmacokinetics and clinical response for artemether–lumefantrine in pregnant and nonpregnant women with uncomplicated plasmodium falciparum malaria in Tanzania. Antimicrob Agents Chemother. 2014;58:4583–92. doi:10.1128/AAC.02595-14.
Tran TH, Day NP, Nguyen HP, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi:10.1056/NEJM199607113350202.
Karbwang J, Tin T, Riichala W, et al. Comparison of artemether and quinine in the treatment of severe falciparum malaria in Southeast Thailand. Trans R Soc Trop Med Hyg. 1995;89:668–71.
Seaton RA, Trevett AJ, Wembri JP, et al. Randomized comparison of intramuscular artemether and intravenous quinine in adult, Melanesian patients with severe or complicated, Plasmodium falciparum malaria in Papua New Guinea. Ann Trop Med Parasitol. 1998;92:133–9.
Myint Pe Than, Shwe Tin. A controlled clinical trial of artemether (qinghaosu derivative) versus quinine in complicated and severe falciparum malaria. Trans R Soc Trop Med Hyg. 1987;81:559–61.
Win K, Than M, Thwe Y. Comparison of combinations of parenteral artemisinin derivatives plus oral mefloquine with intravenous quinine plus oral tetracycline for treating cerebral malaria. Bull World Health Organ. 1992;70:777–82.
The Artemether–Quinine Meta-analysis Study Group. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–50.
Tarning J, Rijken MJ, McGready R, et al. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother. 2012;56:1997–2007. doi:10.1128/AAC.05756-11.
Sangaré LR, Weiss NS, Brentlinger PE, et al. Patterns of anti-malarial drug treatment among pregnant women in Uganda. Malar J. 2011;10:152. doi:10.1186/1475-2875-10-152.
McGready R, Keo NK, Villegas L, et al. Artesunate-atovaquone–proguanil rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Trans R Soc Trop Med Hyg. 2003;97:592–4.
Tagbor HK, Chandramohan D, Greenwood B. The safety of amodiaquine use in pregnant women. Expert Opin Drug Saf. 2007;6:631–6.
Kaye DK, Nshemerirwe R, Mutyaba TS, Ndeezi G. A randomized clinical trial comparing safety, clinical and parasitological response to artemether–lumefantrine and chlorproguanil-dapsone in treatment of uncomplicated malaria in pregnancy in Mulago hospital, Uganda. J Infect Dev Ctries. 2008;2:135–9.
Mcgready R, Ashley EA, Moo E, et al. A randomized comparison of artesunate- atovaquone–proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. J. Infect. Dis. 2005;846–853.
Manyando C, Kayentao K, D’Alessandro U, et al. A systematic review of the safety and efficacy of artemether–lumefantrine against uncomplicated Plasmodium falciparum malaria during pregnancy. Malar J. 2012;11:141. doi:10.1186/1475-2875-11-141.
Bhattacharya PC, Pai-dhungat AJ, Patel K. Artemether in moderate to severe malaria: a multicenter trial in India. Southeast Asian J Trop Med Public Health. 1997;28:736–40.
Bunnag D, Karbwang J, Chitamas S, Harinasuta T. Intramuscular artemether in female patients with uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 1993;24:49–52.
Karbwang J, Na-Bangchang K, Wattanakoon Y, et al. Artemether 5 versus 7 day regimen for severe falciparum malaria. Southeast Asian J Trop Med Public Health. 1994;25:702–6.
Vinh H, Huong N, Ha T, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Trans R Soc Trop Med Hyg. 1997;91:465–7.
Phu NH, Tuan PQ, Day N, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 2010;9:97. doi:10.1186/1475-2875-9-97.
Salako LA, Sowunmi A. Disposition of quinine in plasma, red blood cells and saliva after oral and intravenous administration to healthy adult Africans. Eur J Clin Pharmacol. 1992;42:171–4.
Colley JC, Edwards JA, Heywood R, Purser D. Toxicity studies with quinine hydrochloride. Toxicology. 1989;54:219–26.
Tanimura T. Effects on macaque embryos of drugs reported or suspected to be teratogenic to humans. Acta Endocrinol Suppl (Copenh). 1972;166:293–308.
Mosha D, Mazuguni F, Mrema S, et al. Safety of artemether–lumefantrine exposure in first trimester of pregnancy: an observational cohort. Malar J. 2014;13:197. doi:10.1186/1475-2875-13-197.
Phillips RE, Looareesuwan S, White NJ, et al. Quinine pharmacokinetics and toxicity in pregnant and lactating women with falciparum malaria. Br J Clin Pharmacol. 1986;21:677–83.
Kloprogge F, Jullien V, Piola P, et al. Population pharmacokinetics of quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. J Antimicrob Chemother. 2014;. doi:10.1093/jac/dku228.
Abdelrahim II, Adam I, Elghazali G, et al. Pharmacokinetics of quinine and its metabolites in pregnant Sudanese women with uncomplicated Plasmodium falciparum malaria. J Clin Pharm Ther. 2007;32:15–9. doi:10.1111/j.1365-2710.2007.00788.x.
Kayentao K, Guirou EA, Doumbo OK, et al. Preliminary study of quinine pharmacokinetics in pregnant women with malaria-HIV co-infection. Am J Trop Med Hyg. 2014;90:530–4. doi:10.4269/ajtmh.13-0655.
Krishnan A, Karnad DR. Severe falciparum malaria: an important cause of multiple organ failure in Indian intensive care unit patients. Crit Care Med. 2003;31:2278–84. doi:10.1097/01.CCM.0000079603.82822.69.
Looareesuwan S, White NJ, Silamut K, et al. Quinine and severe falciparum malaria in late pregnancy. Acta Leiden. 1987;55:115–20.
White NJ, Warrell DA, Chanthavanich P, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–6. doi:10.1056/NEJM198307143090201.
Fungladda W, Honrado ER, Thimasarn K, et al. Compliance with artesunate and quinine + tetracycline treatment of uncomplicated falciparum malaria in Thailand. Bull World Health Organ. 1998;76(Suppl 1):59–66.
Adegnika AA, Breitling LP, Agnandji ST, et al. Effectiveness of quinine monotherapy for the treatment of Plasmodium falciparum infection in pregnant women in Lambaréné, Gabon. Am J Trop Med Hyg. 2005;73:263–6.
Achan J, Tibenderana JK, Kyabayinze D, et al. Effectiveness of quinine versus artemether–lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ. 2009;339:b2763.
Gilman A, Rall T, Nies A, Taylor P (1990) Goodman and Gilman’s the pharmacological basis of therapeutics, 8th ed. 993.
McGregor JA, French JI, Parker R, et al. Prevention of premature birth by screening and treatment for common genital tract infections: results of a prospective controlled evaluation. Am J Obstet Gynecol. 1995;173:157–67.
Miller LH, Glew RH, Wyler DJ, et al. Evaluation of clindamycin in combination with quinine against multidrug-resistant strains of Plasmodium falciparum. Am J Trop Med Hyg. 1974;23:565–9.
Kremsner PG. Clindamycin in malaria treatment. J Antimicrob Chemother. 1990;25:9–14.
Calvert C, Ronsmans C. The contribution of HIV to pregnancy-related mortality: a systematic review and meta-analysis. AIDS. 2013;27:1631–9. doi:10.1097/QAD.0b013e32835fd940.
Ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:41–54.
Idemyor V. Human immunodeficiency virus (HIV) and malaria interaction in sub-Saharan Africa: the collision of two Titans. HIV Clin Trials 8:246–53. doi: 10.1310/hct0804-246.
Khoo S, Back D, Winstanley P. The potential for interactions between antimalarial and antiretroviral drugs. AIDS. 2005;19:995–1005.
Berhane K, Karim R, Cohen MH, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37:1245–52.
Brentlinger PE, Behrens CB, Micek MA. Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa. Lancet Infect Dis. 2006;6:100–11. doi:10.1016/S1473-3099(06)70383-8.
Okebe J, Eisenhut M. Pre-referral rectal artesunate for severe malaria. Cochrane Database Syst Rev. 2014;(5). doi:10.1002/14651858.CD009964.pub2.
Newton PN, McGready R, Fernandez F, et al. Manslaughter by fake artesunate in Asia: will Africa be next? PLoS Med. 2006;3:e197. doi:10.1371/journal.pmed.0030197.
Institute of Medicine. Countering the problem of falsified and substandard drugs. Washington, DC: National Academy of Sciences; 2013.
Nayyar GML, Breman JG, Newton PN, Herrington J. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis. 2012;12:488–96. doi:10.1016/S1473-3099(12)70064-6.
World Health Organization. Prequalification of medicines by WHO. 2014. http://www.who.int/mediacentre/factsheets/fs278/en/. Accessed 1 Sept 2014.
Medicines for Malaria Venture. Guilin Pharmaceutical: the world’s first producer of WHO prequalified artesunate for injection for severe malaria. 2014. http://www.apps.who.int/prequal/. Accessed 1 Sept 2014.
Kovacs S, Hawes SE, Maley SN, et al. Technologies for detecting falsified and substandard drugs in low and middle-income countries. PLoS One. 2014;9:e90601. doi:10.1371/journal.pone.0090601.
Lubell Y, Yeung S, Dondorp AM, et al. Cost-effectiveness of artesunate for the treatment of severe malaria. Trop Med Int Health. 2009;14:332–7. doi:10.1111/j.1365-3156.2009.02227.x.
Honrado ER, Fungladda W, Kamoiratanaku P, et al. Cost-effectiveness analysis of artesunate and quinine + tetracycline for the treatment of uncomplicated falciparum malaria in Chanthaburi, Thailand. Bull World Health Organ. 1999;77:235–43.
Binagwaho A, Bate R, Gasana M, et al. Combatting substandard and falsified medicines: a view from Rwanda. PLoS Med. 2013;10:e1001476. doi:10.1371/journal.pmed.1001476.
Centers for Disease Control and Prevention (2013) Treatment of Malaria (Guidelines For Clinicians). 1–8. http://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf. Accessed 1 Sept 2014.
Heist EK, Ruskin JN. Drug-induced arrhythmia. Circulation. 2010;122:1426–35. doi:10.1161/CIRCULATIONAHA.109.894725.
White NJ, Looareesuwan S, Warrell D. Quinine and quinidine: a comparison of EKG effects during the treatment of malaria. J Cardiovasc Pharmacol. 1983;5:173–5.
Royal College of Obsetricians and Gynaecologists (RCOG). The diagnosis and treatment of malaria in pregnancy. London: Royal College of Obstetricians and Gynaecologists; 2010. p. 29.
Acknowledgments
The authors would like to thank Eva Westerly and Richard Kovacs for their editorial help with this manuscript. Rose McGready, Rick Price, Rini Poespropodjo, and Neeru Singh provided patient data on severe vivax malaria.
Conflicts of interest
Stephanie Kovacs, Andy Stergachis, and Marcus Rijken report no conflicts of interest concerning the contents of this article; all authors have taken due care to ensure the integrity of this work. However, please note that Dr. Andy Stergachis serves on the Medicines for Malaria Venture Access and Product Management Advisory Committee; he does not receive any honoraria for this service, only travel expenses for an annual meeting.
Funding sources
This publication was supported in part by the National Center For Advancing Translational Sciences of the National Institutes of Health under award number TL1TR000422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kovacs, S.D., Rijken, M.J. & Stergachis, A. Treating Severe Malaria in Pregnancy: A Review of the Evidence. Drug Saf 38, 165–181 (2015). https://doi.org/10.1007/s40264-014-0261-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0261-9