Abstract
Introduction
Previously, in a 40-week, randomised, double-blind, placebo-controlled core study comprising three phases (9-week dose confirmation, 5-week open-label dose optimisation and 6-month maintenance of effect) in adults with attention-deficit/hyperactivity disorder (ADHD), methylphenidate modified-release long-acting formulation (MPH-LA) at 40–80 mg/day controlled ADHD symptoms as well as decreased functional impairment with a good tolerability profile (NCT01259492). Here, we report the long-term efficacy and safety from a 26-week, open-label extension phase of the same study (NCT01338818).
Methods
Patients in the extension study (n = 298) initiated treatment with MPH-LA (20 mg/day), up-titrated in increments of 20 mg/week to reach individual patient’s daily optimal dose of 40–80 mg. Adverse events (AEs) and serious adverse events (SAEs) were reported at the end of extension study for events monitored from (1) maintenance of effect phase baseline (core study; 12 months) and (2) extension study baseline (6 months). Mean changes in DSM-IV ADHD Rating Scale (DSM-IV ADHD RS) and Sheehan Disability Scale (SDS) total scores are reported for both the timelines. Efficacy was also evaluated using clinician-rated instruments, namely Clinical Global Impression-Improvement Scale (CGI-I) and Clinical Global Impression-Severity Scale (CGI-S).
Results
No unexpected AEs were reported in the extension study. Incidence of SAEs reported during 6 months and 12 months were similar (0.7 %), and no deaths were reported. No SAEs were considered attributable to the drug at the end of 12 months. There were no reports of patients with QT, QTcB or QTcF >500 ms. The mean improvement in DSM-IV ADHD RS and SDS total scores at the end of 12 months were 0.9 and 1.4 points, respectively; and at the end of 6 months were 7.2 and 4.8, respectively. The proportion of patients with improvement in CGI-S scale was 31.4 % and 52.1 % at the end of 12 and 6 months, respectively. Overall, 69.4 % of patients showed clinical improvement in CGI-I scale at the end of 6 months.
Conclusions
In adult patients with ADHD, use of MPH-LA up to 1 year continued to be well tolerated while maintaining the clinical efficacy.
Similar content being viewed by others
No unexpected adverse events or serious adverse events were observed in adult patients with attention-deficit hyperactivity disorder treated with methylphenidate modified-release long-acting formulation over a period of 1 year. |
No new or unexpected results were observed in these patients with regards to the laboratory findings, vital signs, or ECG. |
Patients maintained symptomatic improvement and a reduction in functional impairment over a period of 1 year. |
1 Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurobehavioural, disabling developmental disorder with strong heritability and an estimated global prevalence of approximately 5 % in children and adolescents [1]. Until the publication of the Diagnostic and Statistical Manual of Mental Disorders III revised edition (DSM-III-R), ADHD was considered to be applicable only to children [2]. Long-term follow-up studies have revealed that ADHD persists during adulthood. In spite of remission, approximately 50–60 % of childhood-onset ADHD persists into adolescence and adulthood [3, 4]. Methylphenidate remains one of the most commonly prescribed medications for the treatment of ADHD in adults because of availability of efficacy and safety data [5–16].
Results from previous studies have indicated long-term efficacy and a good tolerability profile for osmotic-release methylphenidate hydrochloride long-acting (OROS-MPH) in flexible dose range in management of ADHD in adults [17, 18]. Methylphenidate modified-release long-acting formulation (MPH-LA; Ritalin-LA®, Novartis Pharma AG) is currently approved for the treatment of ADHD in children aged 6 years and above in several European countries [19–21]. A 40-week, double-blind, randomised, placebo-controlled, multicentre core study was conducted to evaluate the efficacy and safety of MPH-LA in adults with ADHD [22]. The study reported that MPH-LA was safe and effective in the treatment of ADHD in adults, in a dose range of 40–80 mg/day, and this effect was maintained for at least 6 months. Statistically significant improvement in both DSM-IV ADHD Rating Scale (DSM-IV ADHD RS) and Sheehan Disability Scale (SDS) was observed with MPH-LA treatment, and the safety profile was comparable to that of treatment in children.
Long-term safety and efficacy of MPH-LA in adults with ADHD is not yet reported. In order to address this, a 6-month, open-label, flexible-dose, multicentre extension study to the above described 40-week core study was conducted. Here we report the long-term (1 year; last phase of core study + extension study) efficacy and safety of MPH-LA in adults with ADHD.
2 Methods
The current study concentrates on the last phase of the core study (maintenance of effect phase) and the succeeding extension phase conducted at 48 centres in six countries. The core study was conducted between 24 November 2010 and 7 August 2012, and the extension study was conducted between 12 April 2011 and 5 February 2013. The study protocol of the core and extension trials was designed in accordance with the guidelines on studies in ADHD [23]. Written informed consents were provided by all the enrolled patients. The study protocol and all the amendments were approved by the Institutional Review Board or Independent Ethics Committee (core: NCT01259492; extension: NCT01338818). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
2.1 Study Design
The study design of the core study is described elsewhere [22]. Briefly, this was a 6-month, multicentre, open-label extension of a 40-week, double-blind, randomised, placebo-controlled core study (NCT01259492) that comprised three phases: 9-week dose confirmation phase, 5-week open-label dose optimisation phase and 6-month maintenance of effect phase (Fig. 1a). The 6-month maintenance of effect phase was a double-blind, randomised, placebo-controlled, withdrawal period to evaluate the maintenance effect of MPH-LA in adults with ADHD.
Study design of the core and extension study (a) adapted from core study manuscript [22]. MPH-LA methylphenidate modified-release long-acting formulation
Eligible patients entering the extension study (NCT01338818) were initiated on treatment with MPH-LA 20 mg and up-titrated to their optimal doses (dose at which there was an optimal balance between control of symptoms and adverse effects) of 40, 60 or 80 mg/day in increments of 20 mg/week for the first 3 weeks of the extension study (i.e. week 41–43). This re-titration was necessary to accommodate patients who entered the extension study from the placebo arm of the maintenance of effect phase of the core study. The investigator had the flexibility to readjust the doses as necessary (between weeks 44 and 66) as long as the dose remained in the range of MPH-LA 40–80 mg/day (Fig. 1b).
2.2 Study Participants
The study population consisted of ADHD patients (18–60 years) with confirmed childhood onset according to DSM-IV diagnostic criteria [24]. Inclusion criteria for the core study have been previously described [22]. Adult ADHD patients entering the extension study had either completed the 6-month maintenance of effect phase of the core study or discontinued from the maintenance of effect phase due to lack of therapeutic effect (defined as ≥30 % worsening from the maintenance of effect phase baseline and <30 % remaining improvement from the beginning of the core study, using DSM-IV ADHD RS).
A total of 298 patients entered the extension study and received treatment with MPH-LA (Ritalin LA®, Novartis Pharma AG: a racemic mixture of d- and l-threomethylphenidate modified-release hard capsules). Of these, 156 patients were treatment responders (MPH-LA, n = 132; placebo, n = 24) and 91 patients were treatment non-responders (MPH-LA, n = 46; placebo, n = 45) in the maintenance of effect phase. Treatment non-responders were defined as patients who fulfilled both the lack of therapeutic effect criteria in the maintenance of effect phase of the core study. Of the enrolled patients, 51 patients (MPH-LA, n = 38; placebo, n = 13) had discontinued in the maintenance of effect phase of the core study prior to the implementation of an amendment to the inclusion criteria for the extension study protocol (treatment non-responders were required to fulfil both the lack of therapeutic effect criteria). These 51 patients were classified as ‘missing treatment non-responders’.
Details of exclusion criteria for the core study are provided elsewhere [22]. In summary, for the extension study, patients who had developed or showed evidence of psychiatric, cardiovascular, cerebrovascular, respiratory, hepatic, gastrointestinal, renal, haematology or neoplastic disorders during the course of the core study were excluded from the extension study. In addition, other exclusion criteria included pregnancy, seizures, glaucoma, hyperthyroidism, pheochromocytoma and Tourette’s syndrome or a family history of Tourette’s syndrome. Patients with positive urine drug test or abnormal electrocardiogram at the end of the core study or premature discontinuation visit were also excluded from the study. Patients were not allowed to take rescue medication or psychotropic drugs. Patients undergoing psychological or behavioural therapies for treatment of ADHD should have discontinued them at least 1 month prior to the screening visit. Psychological or behavioural treatments for reasons other than ADHD had to be discontinued 3 months prior to entering the study or kept stable with the same therapist during the whole study.
2.3 Safety Assessments
The primary objective of the extension study was to assess the long-term safety of MPH-LA in adults with ADHD. Safety-related events were monitored in terms of adverse events (AEs) and serious adverse events (SAEs), and their severity and relation to the study drug were assessed. In addition, physical examination, changes in vital parameters such as blood pressure, pulse rate and haematological parameters, clinical chemistry and electrocardiogram were also monitored.
Cumulative safety data for 12 months were collected and analysed from the combined period of the maintenance of effect phase of the core study and the extension study. Safety data for 6 months were collected from the extension study. Safety data have been presented from two different baselines: (1) baseline of the maintenance of effect phase until the end of the extension study (12 months’ data); (2) baseline of the extension phase to end of the extension study (6 months’ data).
2.4 Efficacy Assessments
The secondary objective of the study was to evaluate the long-term efficacy of MPH-LA in adults with ADHD (assessments from the maintenance of effect phase baseline and the extension baseline were recorded). Efficacy was primarily assessed using the DSM-IV ADHD RS and the SDS. DSM-IV ADHD RS is a clinician-rated instrument for assessing the severity of ADHD symptoms; it consists of 18 items directly adapted from the ADHD symptom list according to the DSM-IV [25]. SDS consists of a five-item, self-rated questionnaire that measures the extent to which a patient’s disability due to an illness or health problem (e.g., anxiety disorder, painful conditions, ADHD, depression, etc.) interferes with work/school, social life/leisure activities, and family life/home responsibilities [26].
Secondary efficacy assessments were performed using clinician-rated instruments, namely the Clinical Global Impression-Improvement Scale (CGI-I) and the Clinical Global Impression-Severity Scale (CGI-S). The CGI-I is designed to assess the overall change of illness compared with baseline whereas the CGI-S is designed to assess the patient’s current illness state [27]. The proportion of patients with improvement in the CGI-I and CGI-S scales are reported.
A summary of individual efficacy scales, their sub-scores, assessment time points, scoring criteria, assessment baselines and the improvement criteria are provided in Table 1.
2.5 Statistical Analysis
The study sample size was calculated to ensure exposure of ≥300 patients for 6 months and ≥100 patients for 12 months to get the combined exposure data from core and extension studies in accordance with the International Conference on Harmonisation (ICH) guidelines [28] to assess the long-term safety and efficacy of the treatment with MPH-LA.
The All Extension Patients (AEP) analysis set included all patients who had entered the open-label extension study and received at least one dose of MPH-LA. This analysis set was used for all efficacy and safety analyses (Table 2).
Safety analysis of AEP:
-
for 12 months was conducted based on treatment given in the maintenance of effect phase (MPH-LA or placebo)
-
for 6 months was based on mean daily dose given in the extension study (MPH-LA mean daily dose of ≤40 mg or >40–60 mg or >60 mg).
Efficacy in AEP (for 12 months and 6 months) was evaluated based on:
-
treatment given in the maintenance of effect phase of the core study (MPH-LA or placebo).
For both safety and efficacy parameters, data were summarised using descriptive statistics (n, mean, standard deviation, minimum, median, maximum) for continuous variables and using contingency tables (n, %) for discrete variables.
3 Results
3.1 Patient Disposition and Baseline Demographics
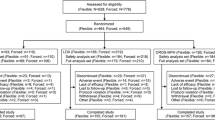
Patient disposition for the core study was described in the core study manuscript [22]. Overall, 298 patients aged 18–60 years with ADHD who had either completed the core study or discontinued from the maintenance of effect phase of the core study (due to lack of therapeutic effect) entered the extension study. Of these, 262 (87.9 %) completed the extension study and 36 (12.1 %) discontinued from the study. The most common reasons for discontinuation were withdrawal of consent by the subjects (3.7 %, n = 11) followed by discontinuation due to AEs (2.7 %, n = 8) (Fig. 2).
Demographics and baseline characteristics are represented based on treatment (MPH-LA or placebo) received during the maintenance of effect phase and were similar for both MPH-LA and placebo patients (Table 3). Among the patients enrolled in the extension study, 160 (53.7 %) were males, and the mean age in the study was 36.3 ± 11.40 years. Most of the patients (n = 272, 91.3 %) were Caucasians.
3.2 Duration of Exposure to Methylphenidate Modified-Release Long-Acting Formulation (MPH-LA)
Of the 298 patients in the extension study, 85 received a mean daily dose of MPH-LA ≤40 mg; 104 patients received >40–60 mg, and 109 patients received >60 mg during the extension study. The overall mean duration of exposure for MPH-LA was 170.5 days in the AEP group, and the mean duration of exposure to the MPH-LA was 155.2, 172.9, and 180.0 days for the ≤40 mg, >40–60 mg, and >60 mg mean daily dose groups, respectively. A total of 125 patients were treated with MPH-LA continuously for the entire duration of 12 months (maintenance of effect phase and extension phase). A total of 136 patients were continuously exposed to MPH-LA for the duration of >365 days, and 354 patients were continuously exposed to MPH-LA for the duration of >180 days throughout the entire core and extension study. The continuous exposure to MPH-LA was compliant with the ICH guidelines [28] to assess the long-term safety and efficacy of treatment with MPH-LA.
3.3 Safety of MPH-LA Over a Continuous Treatment Exposure of up to 12 Months
Overall incidence of AEs from the maintenance of effect phase baseline of the core study to end of extension study was 80.5 % (n = 240) in the AEP set. AEs described here are based on treatment (placebo or MPH-LA) received in the maintenance of effect phase. Overall, the incidence of AEs was comparable between patients receiving placebo (79.3 %, n = 65) and those receiving MPH-LA (81.0 %, n = 175) during the maintenance of effect phase of the core study. Incidence of nasopharyngitis, nausea, upper respiratory tract infection and fatigue was higher (≥5 %) in patients receiving MPH-LA, while the incidence of headache, decreased appetite, dry mouth, diarrhoea, back pain, anxiety, gastroenteritis, oropharyngeal pain, influenza and tachycardia was higher (≥5 %) in patients receiving placebo. SAEs were observed only in about 0.7 % (n = 2) of AEPs from the maintenance of effect phase baseline to the end of the extension study. Both SAEs were observed in the MPH-LA group. One patient reported exostosis of moderate severity on extension day 146, and another patient reported pancreatitis and non-Hodgkin’s lymphoma (pre-existing before enrolment into this study) (Table 4). Neither of these SAEs was suspected to be related to the study medication. No clinically significant observations were noted in haematological or clinical chemistry parameters in the extension study. A single patient reported clinically notable low systolic blood pressure, and five patients (1.7 %) reported clinically notable changes in diastolic blood pressure (four patients with high, one patient with low). Six patients (2.0 %) were reported to have a notable change in heart rate (three patients each with a high and a low rate). Clinically notable changes in weight were recorded in 29 (9.9 %) patients in the AEP. None of the patients had a QT, QTcB or QTcF ≥500 ms. There were minor changes from maintenance of effect baseline in the mean QTc intervals (0.7 ms for QTcB and 1.2 ms for QTcF) in the AEP. There were no clinically meaningful dose-related differences observed between the MPH-LA mean daily doses.
3.4 Safety Profile of MPH-LA Over a Continuous Treatment Exposure of up to 6 Months in the Extension Study
The overall incidence of AEs occurring in the extension study was 69.8 % (n = 208). Incidence of AEs was comparable between MPH-LA mean daily dosage groups (69.4 %, n = 59; 75.0 %, n = 78; and 65.1 %, n = 71 in the ≤40, >40–60 and >60 mg dosage groups, respectively). The most common AE was nasopharyngitis, with an overall incidence of 19.1 % (n = 57). Incidence of nasopharyngitis was higher in the >40–60 mg group (27.9 %) compared with that in the ≤40 and >60 mg groups (17.6 % and 11.9 %, respectively). Headache, decreased appetite, dry mouth and nausea were the other AEs reported, with a frequency of more than 5 % among all patients. No deaths were reported in the study. Two patients receiving a dosage of >60 mg/day reported SAEs; one patient reported exostosis, and the other patient reported pancreatitis and non-Hodgkin’s lymphoma. The exostosis reported was of moderate severity, and the patient recovered with non-drug therapy and continued the study. The patient with pancreatitis and non-Hodgkin’s lymphoma received concomitant medication, with temporary discontinuation of the study drug, and then completed the study. Neither of the SAEs was suspected to be related to the study drug (Table 5).
Three of the extension patients had clinically notable changes in blood pressure: two patients (>60 mg MPH- LA mean daily dose) with high and one patient (>40–60 mg MPH-LA mean daily dose) with low diastolic blood pressure. There were no reports of clinically notable changes in systolic blood pressure. Three patients (1.0 %) receiving a mean daily dose of >60 mg MPH-LA were reported to have a notable increase in heart rate, and 20 patients (6.9 %) showed a clinically notable decrease in weight. None of the patients had a QT, QTcB or QTcF ≥500 ms during the extension phase. There were minor changes from extension baseline in the mean QTc intervals in the AEP (2.4 ms for QTcB and 1.0 ms for QTcF). Patients with mean daily dose of ≤40 mg showed minimal changes in QTcB (−0.6 ms) and QTcF (0.4 ms) intervals during the extension study.
3.5 Efficacy of MPH-LA Over a Continuous Treatment Period of up to 12 Months
The mean improvement in total score of DSM-IV ADHD RS from the maintenance of effect phase baseline to the end of the extension study was 0.9 (Fig. 3). The mean improvement in SDS total score from the maintenance of effect phase baseline to the end of the extension study was 1.4 (Fig. 3). A total of 91 (31.4 %) patients showed improvement in CGI-S score from the maintenance of effect phase baseline to the end of the extension study (MPH-LA, 68 (32.1 %); Placebo, 23 (29.5 %)).
Mean improvement in DSM-IV ADHD RS and SDS total scores from maintenance of effect baseline (12 months data). DSM-IV ADHD RS Diagnostic and Statistical Manual of Mental Disorders IV attention-deficit hyperactivity disorder rating scale, MPH-LA methylphenidate modified-release long-acting formulation, SDS Sheehan Disability Scale
3.6 Efficacy of MPH-LA over a Continuous Treatment of up to 6 Months in the Extension Study
The mean improvement in total score of DSM-IV ADHD RS and SDS from extension baseline to the end of the study was 7.2 and 4.8, respectively (Fig. 4). Overall, 206 (69.4 %) patients showed improvement in CGI-I rating (MPH-LA, 141 (65.3 %); Placebo, 65 (80.2 %)), and 151 (52.1 %) patients showed improvement in CGI-S scale (MPH-LA, 91 (42.9 %); Placebo, 60 (76.9 %)) from the extension study baseline to the end of the extension study.
Mean improvement in DSM-IV ADHD RS and SDS total scores from extension baseline (6 months data). DSM-IV ADHD RS Diagnostic and Statistical Manual of Mental Disorders IV attention-deficit hyperactivity disorder rating scale, MPH-LA methylphenidate modified-release long-acting formulation, SDS Sheehan Disability Scale
4 Discussion
Results of this extension study, combined with the results of the 6-month maintenance of effect phase of the core study, showed that MPH-LA at a dose of 40–80 mg/day administered once daily in adult patients with ADHD is safe and maintains efficacy up to a period of 1 year. The overall incidences of AEs reported in the maintenance of effect phase or the extension phase were comparable between patients receiving MPH-LA and placebo in the maintenance of effect phase of the core study, and the safety profile of MPH-LA did not change with the longer duration of treatment. No consistent relationship was observed between the mean daily dose of MPH-LA and the incidence of AEs. The flexible dosing regimen applied in the extension study allowed titration of MPH-LA dose, such that every individual patient received the dose at which an optimal balance between control of symptoms and adverse effects was achieved. There were no unexpected AEs or SAEs reported in this study. The most common AEs were nasopharyngitis, headache, nausea, decreased appetite and dry mouth, which were similar to those reported in previous studies with other formulations of MPH both in children and adults with ADHD [13, 29–31]. These findings will help address the limited data availability on the long-term safety of MPH in the treatment of adults with ADHD [32].
The proportion of patients receiving MPH-LA who discontinued the extension study due to AEs was 2.3 %, which is much lower than the 18.5 % as reported in a similar 1-year, open-label study on safety of OROS-MPH (36–108 mg/day) in the treatment of adult ADHD patients [17] and the 14.7 % in another 6-month, open-label study on safety and efficacy of dexmethylphenidate extended release (d-MPH-ER) (20–40 mg/day) [29]. The reason for a higher proportion of patients discontinuing due to AEs in these two studies was reported to be dose related, thus necessitating dose reductions in these patients. We suggest that the lower incidence of discontinuation related to MPH-LA in this study can be attributed to the flexible, optimised titration of MPH-LA daily dose in the open-label extension study, thus ascertaining an optimal balance between control of symptoms and AEs.
Overall, only two (0.7 %) patients in the >60 mg dose group reported SAEs, neither of which were considered to be related to the study drug. The incidence of SAEs in this study was lower than that observed in a similar open-label, dose titration, 1-year flexible dose study (36–108 mg/day OROS-MPH) that assessed the safety of OROS-MPH. In that study, 1.5 % of patients reported SAEs, none of which were considered to be related to the study drug [17]. None of the patients reported QT, QTcB or QTcF >500 ms. Overall, the modest change in vital signs and electrocardiogram (ECG) parameters in patients treated with MPH is not considered to be clinically meaningful and is thought to be consistent with the known effects of stimulant medications in ADHD [32].
The results of this extension study suggest improvement in ADHD symptoms and reduction in functional impairment as measured by the DSM-IV ADHD RS, SDS and CGI scales. Mean improvement in total scores of DSM-IV ADHD RS and SDS from the maintenance of effect phase baseline to the end of the extension study were 0.9 and 1.4 points, respectively, confirming that efficacy is maintained over a period of 1 year. Patients who received MPH-LA in the maintenance of effect phase and who continued therapy in the extension study maintained the efficacy as seen in DSM-IV ADHD RS and SDS total scores at the end of the extension study. Patients who received placebo compared with those who received MPH-LA in the maintenance of effect phase showed marked improvement in their DSM-IV ADHD RS and SDS total scores after receiving MPH-LA in the extension study. These results were comparable with another open-label, 6-month, extension study in which there was marked clinical improvement in DSM-IV ADHD RS scores in patients who were switched from placebo to d-MPH-ER (20–40 mg/day), and the benefit was sustained in patients who were already receiving MPH [29]. Similarly, mean improvement in the SDS total score (4.8) in this study was comparable with a 7-week, open-label extension study in which the improvement in SDS total score was 2.8 ± 6.0 and 4.6 ± 5.8 for patients receiving OROS-MPH (18–72 mg/day) and placebo, respectively, in the double-blind period of the study [33].
At the end of the extension study, clinical improvement in CGI-I rating was noted in 141 (65.3 %) and 65 (80.2 %) patients treated (in the maintenance of effect phase) with MPH-LA and placebo, respectively. Although comparison with other studies is difficult due to patient variability influencing response, the response rates reported in this study are similar to those observed in the literature. In a 6-month extension study, 95.1 % of patients maintained on d-MPH-ER (20–40 mg/day) and 95.0 % of patients who switched from placebo to d-MPH-ER showed improvement in CGI-I [28]. In another 1-year study, 81 % improvement was observed in CGI-I scores in patients with ADHD treated with MPH-ER (30–100 mg/day) [31]. Likewise, improvement in the CGI-S scale observed in 42.9 % and 76.9 % of patients who received MPH-LA and placebo treatment (in the maintenance of effect phase), respectively, was similar to that reported in other studies [29, 33].
Overall, the results indicate that MPH-LA at a flexible dose of 40–80 mg/day maintained long-term efficacy over a period of 1 year as observed in the improvement of both ADHD symptoms and functional impairment, as measured by the DSM-IV ADHD RS, SDS and CGI scales.
5 Strengths and Limitations
The key strength of this study was that dosing in the open-label extension study was flexible and obtained by titrating MPH-LA daily dose after ascertaining the optimal balance between control of symptoms and AEs, which closely reflects clinical practice. There is also a limitation in the study design, as it is not a true switch study of patients receiving MPH-LA or placebo switched to MPH-LA optimal dose in the extension study, as all the patients had received MPH-LA before entering the maintenance of effect phase and a sham response was seen in placebo patients. Another limitation could be that not all the patients who finished the study had received MPH-LA continuously for 1 year, and, in these patients, exposure to MPH-LA was interrupted. On the other hand, this interrupted exposure could also be considered a strength of the study and be relevant in terms of ecological validity, since it may reflect real-life exposure.
6 Conclusion
This study confirms the long-term safety and efficacy of MPH-LA for treatment of adults with ADHD, which is comparable to the safety events reported in the core study and other studies of MPH-LA in children with ADHD. No new safety event was reported upon long-term exposure to MPH-LA. The safety and efficacy profile of MPH-LA is also similar to that of other formulations of MPH used in the treatment of adults with ADHD.
References
Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, rev. 50. 1987.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision: DSM-IV-TR Arlington. http://dsm.psychiatryonline.org/book.aspx?bookid=22. Accessed 6 Nov 2012.
Zhang S, Faries DE, Vowles M, et al. ADHD Rating Scale IV: psychometric properties from a multinational study as a clinician-administered instrument. Int J Methods Psychiatr Res. 2005;14:186–201.
Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105.
Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare; 1976. (Data on file).
ICH Guideline E1. The extent of population exposure to assess clinical safety for drugs intended for long-term treatment of non-life threatening conditions. CHMP/EWP/375/95.
Adler LA, Spencer T, McGough JJ, et al. Long-term effectiveness and safety of dexmethylphenidate extended-release capsules in adult ADHD. J Atten Disord. 2009;12:449–59.
Buitelaar JK, Trott GE, Hofecker M, et al. Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD. Int J Neuropsychopharmacol. 2012;15:1–13.
Wender PH, Reimherr FW, Marchant BK, et al. A one year trial of methylphenidate in the treatment of ADHD. J Atten Disord. 2011;15:36–45.
Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205.
Buitelaar JK, Casas M, Philipsen A, et al. Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate. Psychol Med. 2012;42:195–204.
Ref Type: Generic
Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–65.
Lara C, Fayyad J, de GR et al. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry 2009;65:46–54.
Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15:476–95.
Castells X, Ramos-Quiroga JA, Rigau D, et al. Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs. 2011;25:157–69.
Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24:24–9.
Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–63.
Kendall T, Taylor E, Perez A, et al. Diagnosis and management of attention-deficit/hyperactivity disorder in children, young people, and adults: summary of NICE guidance. BMJ. 2008;337:a1239.
Koesters M, Becker T, Kilian R, et al. Limits of meta-analysis: methylphenidate in the treatment of adult attention-deficit hyperactivity disorder. J Psychopharmacol. 2009;23:733–44.
Kooij JJ, Burger H, Boonstra AM, et al. Efficacy and safety of methylphenidate in 45 adults with attention-deficit/hyperactivity disorder. A randomized placebo-controlled double-blind cross-over trial. Psychol Med. 2004;34:973–82.
Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67.
Medori R, Ramos-Quiroga JA, Casas M, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:981–9.
Meszaros A, Czobor P, Balint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137–47.
Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197:1–11.
Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:456–63.
Adler LA, Orman C, Starr HL, et al. Long-term safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: an open-label, dose-titration, 1-year study. J Clin Psychopharmacol. 2011;31:108–14.
Biederman J, Mick E, Surman C, et al. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2010;30:549–53.
Biederman J, Quinn D, Weiss M, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs. 2003;5:833–41.
Coghill D, Banaschewski T, Zuddas A, et al. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237.
Schulz E, Fleischhaker C, Hennighausen K, et al. A double-blind, randomized, placebo/active controlled crossover evaluation of the efficacy and safety of Ritalin (R) LA in children with attention-deficit/hyperactivity disorder in a laboratory classroom setting. J Child Adolesc Psychopharmacol. 2010;20:377–85.
Huss M, Ginsberg Y, Tvedten T, et al. Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial. Adv Ther. 2014;31:44–65.
European Medicines Agency (EMA). Guideline on the clinical investigation of medicinal products for the treatment of attention deficit hyperactivity disorder (ADHD). (EMEA/CHMP/EWP/431734/2008). 2008. Ref Type: Bill/Resolution.
Funding and Acknowledgments
This study was sponsored by Novartis Pharma AG. We thank the patients for their participation and contribution to this study, as well as the investigators and the entire study team. The authors would like to thank Maria Roberts, the trial head employed by Novartis Pharmaceutical Corporation, who contributed to the study design and data collection and was accountable for the delivery of the trial. The authors also acknowledge the contributions of Praveen Duhan, MD for providing valuable feedback and review during the development of the manuscript. The authors would also like to thank Anuradha Nalli Ph.D. (Contract writer funded by Novartis Healthcare Pvt Ltd, India) and Venugopal Peta M. Pharm, Ph.D. (Novartis Healthcare Pvt Ltd, India) for medical writing support. All authors significantly contributed to editing the manuscript for scientific intellectual content. The final draft of the manuscript submitted to the journal was approved by all authors.
Michael Huss provided medical advice, contributed to study design, data collection and interpretation, providing guidance to the development of manuscript. Ylva Ginsberg, Torben Arngrim, Alexandra Philipsen were responsible for data collection, data interpretation, and reviewing and editing the manuscript for intellectual content. Chien-Wei Chen contributed to data analysis and data interpretation. Vinod Kumar contributed to the study design and conduct of the study, as well as data analysis and data interpretation. Preetam Gandhi was the medical specialist for the trial and contributed to the conduct of the study, data analysis and data interpretation and provided direction in the preparation of the manuscript.
Conflicts of interest
Michael Huss is a member of the International Advisory Board of Novartis and has received support for travel to different meetings and payment for lectures. Ylva Ginsberg has served as a consultant and speaker for Novartis and Janssen-Cilag. She has also been a Principal Investigator of two clinical trials funded by Janssen-Cilag, in addition to this trail funded by Novartis. Torben Arngrim has been member of an Advisory Board organized by Novartis in Frankfurt in June 2010. He has been an investigator on the study, ‘MPH-LA in adult patients with ADHD’. Alexandria Philipsen has received consulting fees/fees for participation in review activities from Eli Lilly, Medice, Novartis and Shire. She has received speaker fees and/or travel grants for Eli Lilly, Medice and Shire. Vinod Kumar, Preetam Gandhi and Chien-Wei Chen are employees of Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ginsberg, Y., Arngrim, T., Philipsen, A. et al. Long-Term (1 Year) Safety and Efficacy of Methylphenidate Modified-Release Long-Acting Formulation (MPH-LA) in Adults with Attention-Deficit Hyperactivity Disorder: A 26-Week, Flexible-Dose, Open-Label Extension to a 40-Week, Double-Blind, Randomised, Placebo-Controlled Core Study. CNS Drugs 28, 951–962 (2014). https://doi.org/10.1007/s40263-014-0180-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0180-4