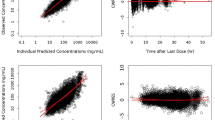
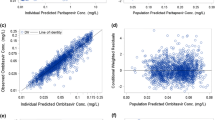
Abstract
Background and Objective
Daclatasvir is a potent, pangenotypic once-daily hepatitis C virus (HCV) NS5A inhibitor that is approved for the treatment of chronic HCV infection. The objective of this analysis was to characterize the pharmacokinetics of daclatasvir in subjects with chronic HCV infection.
Methods
A population pharmacokinetic (PPK) model was developed to evaluate effects of covariates on daclatasvir pharmacokinetics in subjects with chronic HCV infection (n = 2149 from 11 studies). All significant demographic, laboratory, prognostic and treatment covariates (p < 0.05) from univariate screening were included in the full model. The final model was reached by backward elimination (p < 0.001) and simulations were performed to further evaluate the effects of covariates on daclatasvir exposures. The plasma pharmacokinetics of daclatasvir was described by a two-compartment model with linear elimination. Absorption was modeled as a zero-order release followed by a first-order absorption into the central compartment.
Results
The typical value of apparent clearance (CL/F) was 5.7 L/h (1.58% relative standard error [RSE]) and of apparent volume of the central compartment (V c/F) was 58.6 L (2.00% RSE). Modest inter-individual variability was estimated for CL/F (35.1%) and V c/F (29.5%). Statistically significant covariates in the final model were sex, race, virus genotype, baseline creatinine clearance, and alanine aminotransferase (ALT) on CL/F and sex, race, and body weight on V c/F. Covariate effects demonstrated a 30% higher area under the plasma concentration–time curve at steady state (AUCss) in female subjects; effects of all other covariates were <16%.
Conclusions
The model adequately described the daclatasvir pharmacokinetics and estimated relatively small covariate effects. Considering the exposure range for the therapeutic dose of daclatasvir 60 mg once daily and the favorable safety profile, the small difference in exposures due to these covariates is not considered clinically relevant.





Similar content being viewed by others
References
Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3(5):514–20.
United States Food and Drug Administration. FDA approves new treatment for chronic hepatitis C genotype 3 infections. 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455888.htm. Accessed 12 May 2016.
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87.
Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105.
World Health Organization. WHO model list of essential medicines. 19th list. Apr 2015; amended Aug 2015. http://www.who.int/selection_medicines/committees/expert/20/EML_2015_FINAL_amended_AUG2015.pdf?ua=1. Accessed 1 May 2016.
Bifano M, Sevinsky H, Stonier M, Jiang H, Bertz RJ. Daclatasvir, an HCV NS5A replication complex inhibitor, has minimal effect on pharmacokinetics of midazolam, a sensitive probe for cytochrome P450 3A4 [abstract no. O_15]. In: 8th International Workshop on Clinical Pharmacology of Hepatitis Therapy: Cambridge (MA); 26–27 June 2013.
Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54(6):1956–65.
Thanneer N, Roy A, Sukumar P, Bandaru J, Carleen E. Best practices for preparation of pharmacometric analysis data sets. J Pharmacokinet Pharmacodyn. 2014;41:S32.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Chan P, Zhu L, Eley T, Bifano M, Hughes E, Bertz R, et al. Exposure-safety analysis for asunaprevir and daclatasvir in DUAL combination in subjects with hepatitis C virus infection. J Pharmacokinet Pharmacodyn. 2014;41:S41. [Abstracts for conference poster presentations]
Zhu L, Chan P, Eley T, Bifano M, Osawa M, Ueno T, et al. Exposure-efficacy analysis for daclatasvir and asunaprevir in DUAL combination in subjects with genotype 1b hepatitis C virus infection. J Pharmacokinet Pharmacodyn. 2014;41:S38. [Abstracts for conference poster presentations]
Garimella T, Wang R, Luo WL, Hwang C, Sherman D, Kandoussi H, et al. Single-dose pharmacokinetics and safety of daclatasvir in subjects with renal function impairment. Antivir Ther. 2015;20(5):535–43.
Kawamura Y, Akuta N, Sezaki H, Hosaka T, Someya T, Kobayashi M, et al. Determinants of serum ALT normalization after phlebotomy in patients with chronic hepatitis C infection. J Gastroenterol. 2005;40(9):901–6.
Bifano M, Sevinsky H, Persson A, Chung E, Wind-Rotolo M, Hwang C, et al. Single-dose pharmacokinetics of daclatasvir (DCV; BMS-790052) in subjects with hepatic impairment compared with healthy subjects. Hepatology. 2011;54(S1):1004A.
Eley T, Sevinsky H, Huang SP, He B, Zhu K, Kandoussi H, et al. The pharmacokinetics of daclatasvir and asunaprevir administered in combination in studies in healthy subjects and patients infected with hepatitis C virus. Clin Drug Investig. 2014;34(9):661–71.
Eley T, You X, Huang S-P, Symonds W, Li W, Sherman D, et al. Evaluation of drug interaction potential between daclatasvir and sofosbuvir [abstract no. O_14]. In: 8th International workshop on clinical pharmacology of hepatitis therapy: Cambridge (MA); 26–27 June 2013.
Bifano M, Adamczyk R, Hwang C, Kandoussi H, Marion A, Bertz RJ. An open-label investigation into drug-drug interactions between multiple doses of daclatasvir and single-dose cyclosporine or tacrolimus in healthy subjects. Clin Drug Investig. 2015;35(5):281–9.
Bifano M, Sevinsky H, Hwang C, Kandoussi H, Jiang H, Grasela D, et al. Effect of the coadministration of daclatasvir on the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate. Antivir Ther. 2014;19(5):511–9.
Garimella T, Wang R, Luo WL, Wastall P, Kandoussi H, DeMicco M, et al. Assessment of drug-drug interactions between daclatasvir and methadone or buprenorphine-naloxone. Antimicrob Agents Chemother. 2015;59(9):5503–10.
Acknowledgements
The authors would like to thank Russ Wada from Quantitative Solutions for his scientific contributions in data analysis and interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies described in this report were funded by Bristol-Myers Squibb.
Conflict of interest
P. Chan, L. Zhu, R. Bertz, T. Garimella, and M. AbuTarif are all employees of Bristol-Myers Squibb. M. Bifano, T. Eley, and E. Hughes are former employees of Bristol-Myers Squibb and were employed by Bristol-Myers Squibb when the studies and analyses were conducted. M. Osawa and T. Ueno are employees of Bristol-Myers Squibb K.K. Hanbin Li of Quantitative Solutions was funded by Bristol-Myers Squib to carry out the modeling analyses reported in this paper.
Ethical approval
The study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki and were approved by the institutional review board/independent ethics committee at each site.
Informed consent
Written informed consent was obtained from all study participants and/or their legally authorized representative.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chan, P., Li, H., Zhu, L. et al. Population Pharmacokinetic Analysis of Daclatasvir in Subjects with Chronic Hepatitis C Virus Infection. Clin Pharmacokinet 56, 1173–1183 (2017). https://doi.org/10.1007/s40262-016-0504-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0504-2