Abstract
Background
Single nucleotide polymorphisms (SNPs) in the CYP3A5 and CYP3A4 genes have been reported to be an important cause of variability in the pharmacokinetics of tacrolimus in renal transplant patients. The aim of this study was to merge all of the new genetic information available with tacrolimus pharmacokinetics to generate a more robust population model with data from renal transplant recipients.
Methods
Tacrolimus exposure data from 304 renal transplant recipients were collected throughout the first year after transplantation and were simultaneously analyzed with a population pharmacokinetic approach using NONMEM® version 7.2.
Results
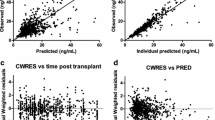
The tacrolimus whole-blood concentration versus time data were best described by a two-open-compartment model with inter-occasion variability assigned to plasma clearance. The following factors led to the final model, which significantly decreased the minimum objective function value (p < 0.001): a new genotype cluster variable combining the CYP3A5*3 and CYP3A4*22 SNPs defined as extensive, intermediate, and poor metabolizers; the standardization of tacrolimus whole blood concentrations to a hematocrit value of 45%; and age included as patients <63 years versus patients ≥63 years. External validation confirmed the prediction ability of the model with median bias and precision values of 1.17 ng/mL (95% confidence interval [CI] –3.68 to 4.50) and 1.64 ng/mL (95% CI 0.11–5.50), respectively. Simulations showed that, for a given age and hematocrit at the same fixed dose, extensive metabolizers required the highest doses followed by intermediate metabolizers and then poor metabolizers.
Conclusions
Tacrolimus disposition in renal transplant recipients was described using a new population pharmacokinetic model that included the CYP3A5*3 and CYP3A4*22 genotype, age, and hematocrit.





Similar content being viewed by others
References
Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant. 2013;13(Suppl 1):11–46.
Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30.
Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–52.
FDA. Draft guidance on tacrolimus 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM181006.pdf. Accessed 07 Sept 2016.
Staatz CE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53.
Astellas Pharma US. Prograf: highlights of prescribing information. https://www.us.astellas.com/docs/prograf.pdf. Accessed 07 Sept 2016.
Wallemacq PE, Furlan V, Möller A, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23:367–70.
Provenzani A, Santeusanio A, Mathis E, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 2013;19:9156–73.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58.
Zahir H, McCaughan G, Gleeson M, et al. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57:298–309.
van Maarseveen EM, Rogers CC, Trofe-Clark J, et al. Drug-drug interactions between antiretroviral and immunosuppressive agents in HIV-infected patients after solid organ transplantation: a review. AIDS Patient Care STDS. 2012;26:568–81.
Mancinelli LM, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69:24–31.
Bekersky I, Dressler D, Mekki QA. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol. 2001;41:176–82.
Jain AB, Venkataramanan R, Cadoff E, et al. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22:57–9.
Thervet E, Anglicheau D, King B, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233–5.
Elens L, Capron A, Kerckhove VV, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873–83.
Renders L, Frisman M, Ufer M, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther. 2007;81:228–34.
Macphee IAM, Fredericks S, Mohamed M, et al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79:499–502.
Zhang X, Liu Z, Zheng J, et al. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 2005;19:638–43.
Hesselink DA, Bouamar R, Elens L, et al. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53:123–39.
Thervet E, Loriot MA, Barbier S, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721–6.
Åsberg A, Midtvedt K, van Guilder M, et al. Inclusion of CYP3A5 genotyping in a nonparametric population model improves dosing of tacrolimus early after transplantation. Transpl Int. 2013;26:1198–207.
Lloberas N, Andreu F, van Gelder T, et al. Impact of CYP3A4*22, CYP3A5*1 and POR*28 polymorphisms on tacrolimus dose optimization and the outcome of kidney transplantation [abstract no. 307]. In: 14th International Congress of Therapeutic Drug Monitoring & Clinical Toxicology; 11–15 Oct 2015; Rotterdam. http://iatdmct2015.org/abstracts/307-2/. Accessed 27 Sept 2016.
Størset E, Holford N, Midtvedt K, et al. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2014;70(1):65–77.
Jacobo-Cabral CO, García-Roca P, Romero-Tejeda EM, et al. Population pharmacokinetic analysis of tacrolimus in Mexican paediatric renal transplant patients: role of CYP3A5 genotype and formulation. Br J Clin Pharmacol. 2015;80(4):630–41.
Musuamba FT, Mourad M, Haufroid V, et al. Time of drug administration, CYP3A5 and ABCB1 genotypes, and analytical method influence tacrolimus pharmacokinetics: a population pharmacokinetic study. Ther Drug Monit. 2009;31:734–42.
Elens L, Bouamar R, Hesselink DA, et al. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin Chem. 2011;57:1574–83.
Zuo XC, Ng CM, Barrett JS, et al. Effects of CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in Chinese adult renal transplant recipients: a population pharmacokinetic analysis. Pharmacogenet Genomics. 2013;23(5):251–61.
Hesselink DA, van Schaik RHN, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54.
Elens L, Capron A, van Schaik RHN, et al. Impact of CYP3A4*22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: toward updated genotype-based dosage guidelines. Ther Drug Monit. 2013;35:608–16.
Elens L, Bouamar R, Shuker N, et al. Clinical implementation of pharmacogenetics in kidney transplantation: calcineurin inhibitors in the starting blocks. Br J Clin Pharmacol. 2014;77:715–28.
Andreu F, Colom H, Grinyó JM, et al. Development of a population PK model of Tacrolimus for adaptive dosage control in stable kidney transplant patients. Ther Drug Monit. 2015;37(2):246–55.
Grinyo JM, Ekberg H, Mamelok RD, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: the Symphony pharmacokinetic substudy. Nephrol Dial Transplant. 2009;24:2269–76.
Capron A, Mourad M, De Meyer M, et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. 2010;11:703–14.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735.
Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–75.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–69.
Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15:1463–8.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12.
Bergstrand M, Hooker AC, Wallin JE, et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28:171–92.
Elens L, van Gelder T, Hesselink DA, et al. CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics. 2013;14:47–62.
Staatz CE, Willis C, Taylor PJ, et al. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72:660–9.
Benkali K, Prémaud A, Picard N, et al. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48:805–16.
Woillard JB, de Winter BC, Kamar N, et al. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations–twice daily Prograf and once daily Advagraf. Br J Clin Pharmacol. 2011;71:391–402.
Moes DJAR, Swen JJ, den Hartigh J, et al. Effect of CYP3A4*22, CYP3A5*3, and CYP3A combined genotypes on cyclosporine, everolimus, and tacrolimus pharmacokinetics in renal transplantation. CPT Pharmacometrics Syst Pharmacol. 2014;3:e100.
Elens L, van Schaik RH, Panin N, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics. 2011;12:1383–96.
Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12:3326–36.
Kuypers DRJ, de Loor H, Naesens M, et al. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenet Genomics. 2014;24:597–606.
Størset E, Åsberg A, Skauby M, et al. Improved tacrolimus target concentration achievement using computerized dosing in renal transplant recipients—a prospective, randomized study. Transplantation. 2015;99:2158–66.
Andrews LM, Riva N, de Winter BC, et al. Dosing algorithms for initiation of immunosuppressive drugs in solid organ transplant recipients. Expert Opin Drug Metab Toxicol. 2015;11:921–36.
Shuker N, Shuker L, van Rosmalen J, et al. A high intrapatient variability in tacrolimus exposure is associated with poor long-term outcome of kidney transplantation. Transpl Int. 2016;29(11):1158–67.
Whalen HR, Glen JA, Harkins V, et al. High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation. 2016;. doi:10.1097/TP.0000000000001129 (Epub 2016 Mar 4).
Vanhove T, Annaert P, Lambrechts D, et al. Effect of ABCB1 diplotype on tacrolimus disposition in renal recipients depends on CYP3A5 and CYP3A4 genotype. Pharmacogenomics J. 2016;. doi:10.1038/tpj.2016.49 (Epub 2016 Jul 5).
Acknowledgements
We are especially grateful to the transplant assistant, Carmen Fernández-Gámiz, for her support in the collection of clinical variables.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nuria Lloberas is a researcher from Instituto de Salud Carlos III Miguel Servet (CP06/00067) and REDinREN RD12/0021/003. This study was supported by Grants from Instituto de Salud Carlos III and Ministerio de Sanidad y Consumo (PI12/01564, PI15/00871), (EC10-144), and Fondo Europeo de Desarrollo Regional (FEDER).
Conflict of Interest
Franc Andreu, Helena Colom, Laure Elens, Teun van Gelder, Ronald H. N. van Schaik, Dennis A. Hesselink, Oriol Bestard, Joan Torras, Josep M. Cruzado, Josep M. Grinyó and Nuria Lloberas declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Andreu, F., Colom, H., Elens, L. et al. A New CYP3A5*3 and CYP3A4*22 Cluster Influencing Tacrolimus Target Concentrations: A Population Approach. Clin Pharmacokinet 56, 963–975 (2017). https://doi.org/10.1007/s40262-016-0491-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0491-3