Abstract
Background and Objective
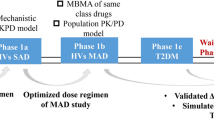
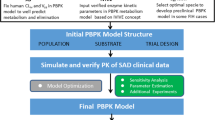
Pharmacokinetic/pharmacodynamic modeling and simulation can aid clinical drug development by dynamically integrating key system- and drug-specific information into predictive profiles. In this study, we propose a methodology to predict pharmacokinetic/pharmacodynamic profiles of sinogliatin (HMS-5552, RO-5305552), a novel glucokinase activator to treat diabetes mellitus, for first-in-patient (FIP) studies.
Methods and Results
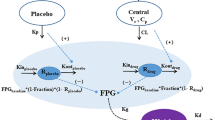
Initially, pharmacokinetic/pharmacodynamic profiles of sinogliatin and another glucokinase activator (US2) previously acquired from healthy subjects were fitted using Model A incorporating an indirect response mechanism. The pharmacokinetic/pharmacodynamic profiles of US2 in patients with type 2 diabetes mellitus (T2DM) were then fitted using Model B incorporating circadian rhythm and food effects after thoughtful research on the difference between healthy subjects and T2DM patients. The differences in results between the two US2 modeling populations were used to scale the values of the pharmacodynamic parameters and refine the pharmacodynamic model of sinogliatin, which was then utilized to project pharmacokinetic/pharmacodynamic profiles of sinogliatin in T2DM patients after an 8-day simulated treatment. Results showed that the projected pharmacokinetic/pharmacodynamic values of five parameters were within 70–130% of values fitted from observed clinical data while the other two remaining projected parameters were within a twofold error. Population pharmacokinetic/pharmacodynamic analysis conducted for sinogliatin also suggested that age and sex were significantly correlated to pharmacokinetic/pharmacodynamic characteristics. Additionally, Model B was combined with a glycosylated hemoglobin (HbA1c) compartment to form Model C, which was then used to project serum HbA1c levels in patients after a 1-month simulated treatment of sinogliatin. The predicted HbA1c changes were nearly identical to observed clinical values (0.82 vs. 0.78%).
Conclusions
Model-based drug development methods utilizing a learn–research–confirm cycle may accurately project pharmacokinetic/pharmacodynamic profiles of new drugs in FIP studies.






Similar content being viewed by others
References
Mager DE, Jusko WJ. Development of translational pharmacokinetic-pharmacodynamic models. Clin Pharmacol Ther. 2008;83:909–12.
Danhof M, de Lange EC, Della POE, et al. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol Sci. 2008;29:186–91.
Jonsson S, Henningsson A, Edholm M, et al. Role of modelling and simulation: a European regulatory perspective. Clin Pharmacokinet. 2012;51:69–76.
Kowalski KG, Olson S, Remmers AE, et al. Modeling and simulation to support dose selection and clinical development of SC-75416, a selective COX-2 inhibitor for the treatment of acute and chronic pain. Clin Pharmacol Ther. 2008;83:857–66.
Peng JZ, Denney WS, Musser BJ, et al. A semi-mechanistic model for the effects of a novel glucagon receptor antagonist on glucagon and the interaction between glucose, glucagon, and insulin applied to adaptive phase II design. AAPS J. 2014;16:1259–70.
Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416.
Bedoya FJ, Matschinsky FM, Shimizu T, et al. Differential regulation of glucokinase activity in pancreatic islets and liver of the rat. J Biol Chem. 1986;261:10760–4.
Grewal AS, Sekhon BS, Lather V. Recent updates on glucokinase activators for the treatment of type 2 diabetes mellitus. Mini Rev Med Chem. 2014;14:585–602.
Xu HR, Sheng L, Chen WL, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: results from a first-in-human single ascending dose study. Drug Des Devel Ther. 2016;10:1619–26.
D’Argenio DZ, Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979;9:115–34.
Liu D, Yang H, Jiang J, et al. Pharmacokinetic and pharmacodynamic modeling analysis of intravenous esomeprazole in healthy volunteers. J Clin Pharmacol. 2016;56:816–26.
Bowen HF, Moorhouse JA. Glucose turnover and disposal in maturity-onset diabetes. J Clin Invest. 1973;52:3033–45.
Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45:1044–50.
Landersdorfer CB, Jusko WJ. Pharmacokinetic/pharmacodynamic modelling in diabetes mellitus. Clin Pharmacokinet. 2008;47:417–48.
Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37:1015–51.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311 (discussion 312–313).
Green B, Duffull S. Caution when lean body weight is used as a size descriptor for obese subjects. Clin Pharmacol Ther. 2002;72:743–4.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64.
Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26.
Rohatagi S, Carrothers TJ, Jin J, et al. Model-based development of a PPARgamma agonist, rivoglitazone, to aid dose selection and optimize clinical trial designs. J Clin Pharmacol. 2008;48:1420–9.
Betts AM, Clark TH, Yang J, et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther. 2010;333:2–13.
Liu D, Ma X, Liu Y, et al. Quantitative prediction of human pharmacokinetics and pharmacodynamics of imigliptin, a novel DPP-4 inhibitor, using allometric scaling, IVIVE and PK/PD modeling methods. Eur J Pharm Sci. 2016;89:73–82.
Claret L, Zheng J, Mercier F, et al. Model-based prediction of progression-free survival in patients with first-line renal cell carcinoma using week 8 tumor size change from baseline. Cancer Chemother Pharmacol. 2016;78:605–10.
Wang Y, Zhu R, Xiao J, et al. Short-term efficacy reliably predicts long-term clinical benefit in rheumatoid arthritis clinical trials as demonstrated by model-based meta-analysis. J Clin Pharmacol. 2016;56:835–44.
Feng S, Shi J, Parrott N, et al. Combining ‘bottom-up’ and ‘top-down’ methods to assess ethnic difference in clearance: bitopertin as an example. Clin Pharmacokinet. 2016;55:823–32.
Jadhav PR, Cook J, Sinha V, et al. A proposal for scientific framework enabling specific population drug dosing recommendations. J Clin Pharmacol. 2015;55:1073–8.
Zager MG, Kozminski K, Pascual B, et al. Preclinical PK/PD modeling and human efficacious dose projection for a glucokinase activator in the treatment of diabetes. J Pharmacokinet Pharmacodyn. 2014;41:127–39.
Schneck KB, Zhang X, Bauer R, et al. Assessment of glycemic response to an oral glucokinase activator in a proof of concept study: application of a semi-mechanistic, integrated glucose-insulin-glucagon model. J Pharmacokinet Pharmacodyn. 2013;40:67–80.
Radziuk J, Pye S. Quantitation of basal endogenous glucose production in Type II diabetes: importance of the volume of distribution. Diabetologia. 2002;45:1053–84.
Hong J, Gu WQ, Zhang YF, et al. The interplay of insulin resistance and beta-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine. 2007;31:93–9.
Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour. Diabetologia. 2006;49:1619–28.
Radziuk J, Pye S. Production and metabolic clearance of glucose under basal conditions in Type II (non-insulin-dependent) diabetes mellitus. Diabetologia. 2001;44:983–91.
Roge RM, Klim S, Kristensen NR, et al. Modeling of 24-hour glucose and insulin profiles in patients with type 2 diabetes mellitus treated with biphasic insulin aspart. J Clin Pharmacol. 2014;54:809–17.
Shapiro ET, Polonsky KS, Copinschi G, et al. Nocturnal elevation of glucose levels during fasting in noninsulin-dependent diabetes. J Clin Endocrinol Metab. 1991;72:444–54.
Dalla MC, Caumo A, Basu R, et al. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–43.
Dalla MC, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49:419–29.
Jauslin PM, Silber HE, Frey N, et al. An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol. 2007;47:1244–55.
Martin J. Red blood cell physiology. Biomed Instrum Technol. 1995;29:150–1.
Matschinsky FM, Zelent B, Doliba N, et al. Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care. 2011;34(Suppl 2):S236–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by Ministry of Science and Technology of the People’s Republic of China (the ‘12th Five-year’ National Key Technology R&D Program of China; No. 2012ZX09303006-002 and 2014ZX09101002-004), the National Natural Science Foundation of China (No. 81403013), and the Shanghai Science and Technology Committee (the Shanghai Technology Program, No. 15XD1520500).
Conflict of interest
Yi Zhang, John Choi, and Li Chen are employees in HuaMedicine (Shanghai) Ltd.), the company developing HMS-5552. Dongyang Liu, Ji Jiang, Xuening Li, Dalong Zhu, Dawei Xiao, Yanhua Ding, Hongwei Fan and Pei Hu declare no conflicts of interest that might be are relevant to the contents of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, D., Zhang, Y., Jiang, J. et al. Translational Modeling and Simulation in Supporting Early-Phase Clinical Development of New Drug: A Learn–Research–Confirm Process. Clin Pharmacokinet 56, 925–939 (2017). https://doi.org/10.1007/s40262-016-0484-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0484-2