Abstract
Background and Objective
Losmapimod is an orally available, potent p38 mitogen-activated protein kinase inhibitor. It is in development as an anti-inflammatory drug in different therapeutic areas. Clinical studies have shown that losmapimod is well tolerated and safe in humans and several studies have shown its pharmacological effect in the target diseases. The aim of this work was to develop a population pharmacokinetic model and to explore the covariates affecting the pharmacokinetics of losmapimod using data collected from healthy volunteers and patients with rheumatoid arthritis (RA) and chronic obstructive pulmonary diseases (COPD).
Subjects and methods
The plasma concentration data were pooled from four of the losmapimod clinical studies, with 30 healthy subjects, 23 subjects with RA and 24 subjects with COPD. Non-linear mixed–effects modelling was used to build a base model to characterize the structure and describe the variability of the pharmacokinetics of losmapimod. The available demographic covariates were explored to further explain the inter-subject variability. New data generated from another RA study with 34 subjects were used to validate the final model. All modelling was conducted using NONMEM® VI.
Results
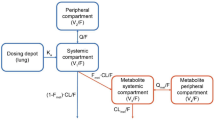
A two-compartment model with first-order elimination and time-dependent first-order absorption was found to best fit the concentration–time profiles of losmapimod following oral administration. The first phase of the absorption was more variable than the second phase for most of the subjects in the dataset. There was no apparent difference in the structure of the pharmacokinetic model among healthy subjects and patients with RA and COPD. A slightly higher residual error was associated with COPD patients compared with the healthy and RA subjects. Three demographic covariates, namely sex, age and bodyweight, were retained in the final model to explain the inter-individual variability on pharmacokinetic parameters apparent total oral clearance (CL/F), apparent volume of distribution of the central compartment (V1) and the first-order absorption rate constant (ka). Most of the fixed effect parameters (θpop,j and θ for covariate) of the final model were estimated with good precision (% relative standard error [RSE] ≤30 %). The inter-individual variability was precisely estimated (%RSE ≤30 %) for clearance and ka, but less precise for inter-compartmental clearance and volumes of distribution. The mean clearance following oral administration of losmapimod was approximately 31.2 L/h (95 % CI 27.7–35.2) for males and 24.6 L/h (95 % CI 22.1–27.3) in females.
Conclusion
The population pharmacokinetic model described in this work well characterized the pharmacokinetic profile of losmapimod following administration of a single oral dose and repeated oral doses in healthy subjects and patients with RA and COPD. Although sex, bodyweight and age were significant factors influencing some pharmacokinetic parameters of losmapimod, the relatively small magnitude of the effect did not result in concerns for dose adjustment.







Similar content being viewed by others
References
Schieven GL. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem. 2005;5:921–8.
Schieven GL. The p38a kinase plays a central role in inflammation. Curr Top Med Chem. 2009;9:1038–48.
Goldstein DM, Kuglstatter A, Lou Y, et al. Selective p38r inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J Med Chem. 2010;53:2345–53.
Aston NM, Bamborough P, Buckton JB, et al. p38α Mitogen-activated protein kinase inhibitors: optimization of a series of biphenylamides to give a molecule suitable for clinical progression. J Med Chem. 2009;52:6257–69.
GSKClinicalStudyRegister.com identifier: HM2010/00107/00, study no. PM1113022. Evaluation of the safety, tolerability, pharmacokinetics and pharmacodynamics of single intravenous dose(s) and a single oral dose of GW856553 in healthy volunteers. http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=113022&studyId=1E5A07BD-2DA1-492B-B305-38AEBC275A62&compound=losmapimod. Accessed 28 Nov 2012.
ClinicalTrials.gov identifier: NCT01218126. Randomised, double-blind, placebo-controlled, parallel-group, multi-centre, dose ranging study to evaluate the efficacy and safety of losmapimod tablets administered twice daily compared with placebo for 24 weeks in adult subjects with chronic obstructive pulmonary disease (COPD). 2010. http://clinicaltrials.gov/ct2/show/NCT01218126. Accessed 28 Nov 2012.
Lomas DA, Lipson DA, Miller BE, et al. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2012;52(3):416–24.
ClinicalTrials.gov identifier: NCT00633022. A study to evaluate the effects of 3 months dosing with GW856553, as assessed FDG-PET/CT imaging. 2008. http://clinicaltrials.gov/ct2/show/NCT00633022. Accessed 28 Nov 2012.
Lukey PT, Perry HC, Yang S, et al. Single doses of p38 MAP kinase inhibitors and prednisolone affect biomarkers in patients with active rheumatoid arthritis (RA). Open J Immunol. 2012;2(3):85–97.
Cheriyan J, Webb AJ, Sarov-Blat L, et al. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123:515–23.
Beal SL, Sheiner LB, Boeckmann AJ, editors. NONMEM users guides (1989–2006). Ellicott City: ICON Development Solutions; 2006.
Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–66.
Brendel K, Comets E, Laffont C, et al. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37:49–65.
Beal SL, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989–2009). Ellicott City: Icon Development Solutions; 2009.
ClinicalTrials.gov identifier: NCT01541852. Losmapimod in chronic obstructive pulmonary disease patients stratified by fibrinogen (EVOLUTION). http://clinicaltrials.gov. Accessed 28 Nov 2012.
ClinicalTrials.gov identifier: NCT00910962. a study to evaluate the safety of 12 weeks of dosing with GW856553 and its effects on inflammatory markers, infarct size, and cardiac function in subjects with myocardial infarction without ST-segment elevation (Solstice). http://clinicaltrials.gov/ct2/show/NCT01541852. Accessed 28 Nov 2012.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41–67.
ClinicalTrials.gov identifier: NCT00642148. A 12 week study to assess efficacy and safety of GW856553 in subjects with chronic obstructive pulmonary disease (COPD). 2008. http://clinicaltrials.gov/ct2/show/NCT00642148?term=NCT00642148&rank=1. Accessed 28 Nov 2012.
ClinicalTrials.gov identifier: NCT00393146. A study to investigate the effect of 28 days of dosing with GW856553 on patients with rheumatoid arthritis. 2006. http://clinicaltrials.gov/ct2/show/NCT00393146?term=NCT00393146&rank=1. Accessed 28 Nov 2012.
Ogungbenro K, Dokoumetzidis A, Aarons L. Application of optimal design methodologies in clinical pharmacology experiments. Pharm Stat. 2009;8:239–52.
Ogungbenro K, Aarons L. Sample-size calculations for multi-group comparison in population pharmacokinetic experiments. Pharm Stat. 2010;9:255–68.
Yang S, Beerahee M. Power estimation using a population pharmacokinetics model with optimal design by clinical trial simulations: application in pharmacokinetic drug–drug interaction studies. Eur J Clin Pharmacol. 2011;67:225–33.
Acknowledgments
All authors are employees of GlaxoSmithKline. No sources of funding were used to assist in the preparation of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, S., Lukey, P., Beerahee, M. et al. Population Pharmacokinetics of Losmapimod in Healthy Subjects and Patients with Rheumatoid Arthritis and Chronic Obstructive Pulmonary Diseases. Clin Pharmacokinet 52, 187–198 (2013). https://doi.org/10.1007/s40262-012-0025-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0025-6