Abstract
Background
The role of umeclidinium plus vilanterol as a combination therapy for chronic obstructive pulmonary disease (COPD) has not yet been clearly defined.
Objective
The aim of this meta-analysis was to evaluate the efficacy and safety of umeclidinium plus vilanterol, in contrast to either monotherapy or placebo.
Methods
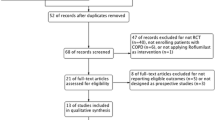
Systematic searches were conducted in Pubmed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and the Chinese Biomedical Literature Database (CBM). Randomized clinical trials pertaining to the treatment of COPD with the combination of umeclidinium and vilanterol, versus umeclidinium, vilanterol or placebo, were reviewed. Studies were pooled to mean differences (MDs), with 95 % confidence intervals (CIs).
Results
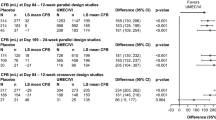
Six studies were included in our meta-analysis. The application of umeclidinium plus vilanterol compared with umeclidinium alone showed increases in trough forced expiratory volume in 1 s [FEV1] (MD 0.05 L, 95 % CI 0.04–0.07; p < 0.00001) and forced vital capacity [FVC] (MD 0.07 L, 95 % CI 0.04–0.10; p < 0.00001). Similarly, versus vilanterol alone, the application of umeclidinium plus vilanterol showed increases in trough FEV1 (MD 0.10, 95 % CI 0.08–0.12; p < 0.00001) and FVC (MD 0.16 L, 95 % CI 0.13–0.20; p < 0.00001). Compared with placebo, umeclidinium plus vilanterol also showed increases in trough FEV1 (MD 0.21 L, 95 % CI 0.19–0.22; p < 0.00001) and FVC (MD 0.31 L, 95 % CI 0.26–0.35; p < 0.00001). In addition, umeclidinium plus vilanterol has beneficial effects on dyspnea, albuterol use, and health-related quality of life compared with the other three groups.
Conclusions
Compared with the other three groups, i.e. placebo, umeclidinium and vilanterol, umeclidinium plus vilanterol improves lung function and quality of life in patients with COPD, reduces the use of albuterol, and does not increase the incidence of adverse events and serious adverse events.






Similar content being viewed by others
References
Global initiative for chronic obstructive lung disease (GOLD 2016). www.goldcopd.org. Accessed 3 May 2016.
Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–67.
Cazzola M, Segreti A, Matera MG. New developments in the combination treatment of COPD: focus on umeclidinium/vilanterol. Drug Des Devel Ther. 2013;7:1201–8.
de Miguel-Diez J, Jimenez-Garcia R. Considerations for new dual-acting bronchodilator treatments for chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2014;23(4):453–6.
Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg C, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145(5):981–91.
Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–86.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Rev Esp Salud Publica. 2000;74(2):107–18.
Higgins JPT, Green S (eds). Cochrane handbook for systematic reviews of interventions. Version 5.0.2. 2009. New York: Wiley. The Cochrane Cllaboration. http://www.cochrane-handbook.org. Accessed June 2008.
Laurendeau C, Pribil C, Perez T, Roche N, Simeoni MC, Detournay B. Validation study of the BDI/TDI scores in chronic obstructive pulmonary disease. Rev Mal Respir. 2009;26(7):735–43.
Wilcox TK, Chen WH, Howard KA, Wiklund I, Brooks J, Watkins ML, et al. Item selection, reliability and validity of the Shortness of Breath with Daily Activities (SOBDA) questionnaire: a new outcome measure for evaluating dyspnea in chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2013;11:196.
Rutten-van Molken M, Roos B, Van Noord JA. An empirical comparison of the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial setting. Thorax. 1999;54(11):995–1003.
Feldman G, Walker RR, Brooks J, Mehta R, Crater G. 28-day safety and tolerability of umeclidinium in combination with vilanterol in COPD: a randomized placebo-controlled trial. Pulm Pharmacol Ther. 2012;25(6):465–71.
Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–46.
Donohue JF, Niewoehner D, Brooks J, O’Dell D, Church A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15:78.
Maltais F, Singh S, Donald AC, Crater G, Church A, Goh AH, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. Ther Adv Respir Dis. 2014;8(6):169–81.
Rodrigo GJ, Neffen H. A systematic review of the efficacy and safety of a fixed-dose combination of umeclidinium and vilanterol for the treatment of COPD. Chest. 2015;148(2):397–407.
Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25.
Schelueter M, Gonzalez-Rojas N, Baldwin M, Groenke L, Voss F, Reason T. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting β2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016;10(2):89–104.
Bateman ED, Mahler DA, Vogelmeier CF, Wedzicha JA, Patalano F, Banerji D. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Rev Respir Med. 2014;8(3):357–79.
Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of umeclidinium/vilanterol in symptomatic COPD Spanish patients. Value Health. 2015;18(7):A500–1.
Punekar YS, Roberts G, Ismaila A, O’Leary M. Cost-effectiveness of umeclidinium/vilanterol combination therapy compared to tiotropium monotherapy among symptomatic patients with chronic obstructive pulmonary disease in the UK. Cost Eff Resour Alloc. 2015;13:22.
Acknowledgments
The authors would like to acknowledge the librarians of Shandong Provincial Hospital Library for their contributions in the search strategies and full-text retrieval.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Li Wang, Chun-juan Zhai, Yao Liu, Yi Liu, Shu-juan Jiang declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Zhai, Cj., Liu, Y. et al. Umeclidinium Plus Vilanterol Versus Placebo, Umeclidinium, or Vilanterol Monotherapies for Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 36, 865–875 (2016). https://doi.org/10.1007/s40261-016-0449-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0449-0