Abstract
Background and Objective
Piperaquine–dihydroartemisinin combination therapy has established efficacy for the treatment of malaria; however, a more comprehensive understanding of the pharmacokinetic properties and factors contributing to inter- and intra-individual variability is critical to optimize clinical use. This study assessed the effects of food on the pharmacokinetics of combination piperaquine–dihydroartemisinin administration in healthy volunteers.
Methods
This was an open-label, single-dose, parallel-group study. Participants were randomly allocated to receive oral piperaquine–dihydroartemisinin either after an overnight fast or immediately after a standardized, high-fat, high-calorie meal. Blood samples were collected for analysis of plasma piperaquine and dihydroartemisinin concentrations, which were utilized for calculation of pharmacokinetic parameters, using a standard model-independent approach.
Results
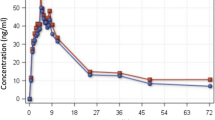
Consumption of a high-fat, high-calorie meal resulted in substantial increases in the extent of exposure to piperaquine (ratio between area under the plasma concentration–time curve [AUC] values from 0 to 168 h in the fed and fasted states [AUC0–168 h FED/AUC0–168 h FASTED] = 299 %, 90 % confidence interval [CI] 239–374 %). This likely reflects an increase in the oral bioavailability of the drug, directly related to the fat content of the meal. Co-administration of food was also found to result in both delayed and enhanced absorption of dihydroartemisinin (ratio between AUC values from time zero to infinity in the fed and states [AUC∞ FED/AUC∞ FASTED] = 142 %, 90 % CI 113–178 %; ratio between mean transit time [MTT] values in the fed and fasted states [MTTFED/MTTFASTED] = 135 %, 90 % CI 114–160 %).
Conclusion
Although food was found to significantly impact on the pharmacokinetics of piperaquine and dihydroartemisinin, given the low fat content of standard meals within endemic regions and the anorexic effects of malaria infection, these results are unlikely to impact on the clinical utility of these drugs. However, co-administration of food with these anti-malarials by populations consuming a typical Western diet should be avoided to reduce the risk of toxic side effects. It is therefore a general recommendation that piperaquine–dihydroartemisinin not be administered within ±3 h of food consumption.




Similar content being viewed by others
References
World Health Organization. Guidelines for the treatment of malaria. 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1. Accessed 27 July 2015.
Ashley EA, McGready R, Hutagalung R, Phaiphun L, Slight T, Proux S, Thwai KL, Barends M, Looareesuwan S, White NJ, Nosten F. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin–piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis. 2005;41(4):425–32.
Myint HY, Ashley EA, Day NPJ, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin–piperaquine. Trans R Soc Trop Med Hyg. 2007;101(9):858–66.
Karema C, Fanello CI, van Overmeir C, van Geertruyden JP, van Doren W, Ngamije D, D’Alessandro U. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans R Soc Trop Med Hyg. 2006;100(12):1105–11.
Smithuis F, Kyaw MK, Phe O, Aye KZ, Htet L, Barends M, Lindegårdh N, Singtoroj T, Ashley E, Lwin S, Stepniewska K, White NJ. Efficacy and effectiveness of dihydroartemisinin–piperaquine versus artesunate–mefloquine in falciparum malaria: an open-label randomised comparison. Lancet. 2006;367(95828):2075–85.
Tran TH, Dolecek C, Pham PM, Nguyen TD, Nguyen TT, Le HT, Dong TH, Tran TT, Stepniewska K, White NJ, Farrar J. Dihydroartemisinin–piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363(9402):18–22.
Binh TQ, Ilett KF, Batty KT, Davis TM, Hung NC, Powell SM, Thu LT, Thien HV, Phuöng HL, Phuöng VD. Oral bioavailability of dihydroartemisinin in Vietnamese volunteers and in patients with falciparum malaria. Br J Clin Pharmacol. 2001;51(6):541–6.
Hung TY, Davis TM, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol. 2004;57(3):253–62.
Na-Bangchang K, Krudsood S, Silachamroon U, Molunto P, Tasanor O, Chalermrut K, Tangpukdee N, Matangkasombut O, Kano S, Looareesuwan S. The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J Trop Med Public Health. 2004;35(3):575–82.
Tarning J, Ashley EA, Lindegårdh N, Stepniewska K, Phaiphun L, Day NP, McGready R, Ashton M, Nosten F, White NJ. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin–piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother. 2008;52(3):1052–61.
European Medicines Agency. Eurartesim: piperaquine tetraphosphate/dihydroartemisinin. Product information: 28/07/2014 Eurartesim -EMEA/H/C/001199 -N/0013. 2014. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001199/human_med_001450.jsp&mid=WC0b01ac058001d124. Accessed 5 Aug 2015.
Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–55.
Chinh NT, Quang NN, Thanh NX, Dai B, Travers T, Edstein MD. Short report: pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am J Trop Med Hyg. 2008;79(4):620–3.
Sim IK, Davis TME, Ilett KF. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother. 2005;46(6):2407–11.
Annerberg A, Lwin KM, Lindegårdh N, Khrutsawadchai S, Ashley E, Day NPJ, Singhasivanon P, Tarning J, White NJ, Nosten N. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob Agents Chemother. 2011;55(9):3971–6.
Hai TN, Hietala SF, Van Huong NV, Ashton M. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 2008;107(2):145–9.
Lwin KM, Phyo AP, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegårdh N, Nosten F. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin–piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56(3):1571–7.
Tarning J, Lindegårdh N, Lwin KM, Annerberg A, Kiricharoen L, Ashley E, White NJ, Nosten F, Day NP. Population pharmacokinetic assessment of the effect of food on piperaquine bioavailability in patients with uncomplicated malaria. Antimicrob Agents Chemother. 2014;58(4):2052–8.
Dien TK, de Vries PJ, Khanh NX, Koopmans R, Binh LN, Duc DD, Kager PA, van Boxtel CJ. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob Agents Chemother. 1997;41(5):1069–72.
Fitoussi S, Thang C, Lesauvage E, Barré J, Charron B, Filali-Ansary A, Lameyre V. Bioavailability of a co-formulated combination of amodiaquine and artesunate under fed and fasted conditions: a randomised, open-label crossover study. Arzneimittelforschung. 2009;59(7):370–6.
Tan B, Naik H, Jang IJ, Yu KS, Kirsch LE, Shin CS, Craft JC, Fleckenstein L. Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects. Malar J. 2009;8:304.
Tarning J, Lindegårdh N, Sandberg S, Day NJ, White NJ, Ashton M. Pharmacokinetics and metabolism of the antimalarial piperaquine after intravenous and oral single doses to the rat. J Pharm Sci. 2008;97(8):3400–10.
Premji ZG, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I, Mulure N, Nwaiwu O, Kokwaro G. The content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether–lumefantrine: a review of the evidence. Malar J. 2008;7:244.
Djimdé A, Lefèvre G. Understanding the pharmacokinetics of Coartem®. Malar J. 2009;8(Suppl 1):S4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was co-funded by Sigma-Tau Farmaceutiche Riunite SpA and the Medicines for Malaria Venture.
Disclosure of potential conflicts of interest
The authors SER, AME and DU declare that they have no conflict of interest. SS, YL and BF are employed by institutions that received funding (commercial sponsorship of contract research) from Sigma-Tau Industrie Farmaceutiche Riunite SpA for the submitted research. GV, AB and SP are employees of Sigma-Tau Industrie Farmaceutiche Riunite SpA.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Reuter, S.E., Evans, A.M., Shakib, S. et al. Effect of Food on the Pharmacokinetics of Piperaquine and Dihydroartemisinin. Clin Drug Investig 35, 559–567 (2015). https://doi.org/10.1007/s40261-015-0312-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0312-8