Abstract
Background and Objectives
ALO-02 capsules, intended to deter abuse, contain pellets of extended-release oxycodone hydrochloride (HCl), an opioid agonist, surrounding sequestered naltrexone HCl, an opioid antagonist. The objective of this study was to determine the effects of administration of ALO-02 with 20 or 40 % ethanol on the pharmacokinetics of oxycodone.
Methods
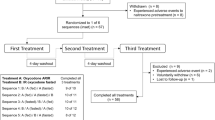
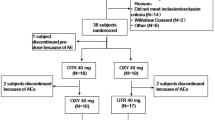
This was an open-label, single-dose, randomized, three-way crossover study in 18 healthy fasting adults administered ALO-02 20/2.4 mg (oxycodone/naltrexone) with water, 20 % ethanol, or 40 % ethanol, each under naltrexone block.
Results
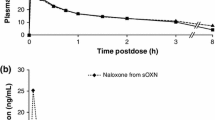
Median time to maximum concentration was 12 h postdose when ALO-02 was administered with water or 20 % ethanol and decreased to 8 h postdose with 40 % ethanol. Geometric mean area under the plasma concentration–time curve (AUC) from time zero extrapolated to infinity (AUC∞) and maximum concentration (C max) values were similar for ALO-02 administered with water or 20 % ethanol, and increased by about 13 and 37 %, respectively, for ALO-02 administered with 40 % ethanol versus water. The 90 % confidence intervals (CIs) for AUC∞ and C max ratios of ALO-02 with 20 % ethanol versus water were within 80–125 %; upper 90 % CIs were >125 % for ALO-02 with 40 % ethanol versus water. The most common adverse events were mild-to-moderate vomiting, nausea, headache, and somnolence. Incidence of adverse events increased for ALO-02 given with ethanol versus water.
Conclusions
Oxycodone exposures (C max) were unaffected when ALO-02 was administered with 20 % ethanol but C max increased by 37 % with 40 % ethanol versus water. ALO-02 administered with ethanol under naltrexone block was generally well tolerated.


Similar content being viewed by others
References
Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9.
White AG. Economic impact of opioid abuse, dependence, and misuse. Am J Pharm Benefits. 2011;3:e59–70.
Mastropietro DJ, Omidian H. Current approaches in tamper-resistant and abuse-deterrent formulations. Drug Dev Ind Pharm. 2013;39:611–24.
Johnson F, Setnik B. Morphine sulfate and naltrexone hydrochloride extended-release capsules: naltrexone release, pharmacodynamics, and tolerability. Pain Physician. 2011;14:391–406.
Setnik B, Goli V, Levy-Cooperman N, Mills C, Shram M, Smith I. Assessing the subjective and physiological effects of intranasally administered crushed extended-release morphine formulations with and without a sequestered naltrexone core in recreational opioid users. Pain Res Manag. 2013;18:e55–62.
Setnik B, Sommerville K, Goli V, Han L, Webster L. Assessment of pharmacodynamic effects following oral administration of crushed morphine sulfate and naltrexone hydrochloride extended-release capsules compared with crushed morphine sulfate controlled-release tablets and placebo in nondependent recreational opioid users. Pain Med. 2013;14:1173–86.
Stauffer J, Setnik B, Sokolowska M, Romach M, Johnson F, Sellers E. Subjective effects and safety of whole and tampered morphine sulfate and naltrexone hydrochloride (ALO-01) extended-release capsules versus morphine solution and placebo in experienced non-dependent opioid users: a randomized, double-blind, placebo-controlled, crossover study. Clin Drug Investig. 2009;29:777–90.
Webster LR, Johnson FK, Stauffer J, Setnik B, Ciric S. Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users. Drugs R D. 2011;11:259–75.
Sommerville KW. Development of a oxycodone/naltrexone combination capsule (ALO-02). Neurotherapeutics. 2013;10:593–600.
Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville: US Department of Health and Human Services; 2013.
Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: volume I. Summary of national findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586 findings). Rockville: US Department of Health and Human Services; 2010.
Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40–54.
Meyer RJ, Hussain AS. Awareness topic: mitigating the risks of ethanol induced dose dumping from oral sustained/controlled release dosage forms. Washington DC: US Food and Drug Administration; 2005.
Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Bull Med Ethics. 2002;182:17–23.
International Conference on Harmonization. E6 Good Clinical Practice. 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 22 Aug 2013.
World Medical Association. Declaration of Helsinki. 2008. http://www.wma.net/en/30publications/10policies/b3/. Accessed 22 Aug 2013.
Center for Drug Evaluation and Research. Food-effect bioavailability and fed bioequivalence studies. Rockville: US Food and Drug Administration; 2002.
US Food and Drug Administration. Information for healthcare professionals: hydromorphone hydrochloride extended-release capsules (marketed as Palladone). In: FDA Alert [7/2005]: Postmarket Drug Safety Information for Patients and Providers. 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm129288.htm. Accessed 19 Aug 2013.
Hendeles L, Wubbena P, Weinberger M. Food-induced dose dumping of once-a-day theophylline. Lancet. 1984;2:1471.
Volpe DA, Asafu-Adjaye EB, Ellison CD, Doddapaneni S, Uppoor RS, Khan MA. Effect of ethanol on opioid drug permeability through caco-2 cell monolayers. AAPS J. 2008;10:360–2.
Wills RJ, Crouthamel WG, Iber FL, Perkal MB. Influence of alcohol on the pharmacokinetics of diazepam controlled-release formulation in healthy volunteers. J Clin Pharmacol. 1982;22:557–61.
Johnson FK, Ciric S, Boudriau S, Kisicki J, Stauffer J. Effects of alcohol on the pharmacokinetics of morphine sulfate and naltrexone hydrochloride extended release capsules. J Clin Pharmacol. 2012;52:747–56.
Malhotra B, Matschke K, Bramson C, Wang Q, Salageanu J. Relative bioavailability study of an abuse-deterrent formulation of extended-release oxycodone with sequestered naltrexone (ALO-02) versus immediate-release oxycodone tablets in healthy volunteers. J Bioequiv Availab. 2014;6:186–91.
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79:461–79.
Acknowledgments
This study was sponsored by Pfizer Inc. All authors are employees of Pfizer Inc. Medical writing support was provided by Mary Kunjappu, PhD, of Engage Scientific Solutions and funded by Pfizer Inc.
Funding
Pfizer Inc.
Conflict of interest
All authors are employees of Pfizer Inc.
Ethical standards
This study was conducted according to principles set forth in the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Guidelines for Good Clinical Practice, and the Declaration of Helsinki. All participants provided informed consent prior to enrollment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov, identifier NCT01677039.
Rights and permissions
About this article
Cite this article
Malhotra, B.K., Matschke, K., Wang, Q. et al. Effects of Ethanol on the Pharmacokinetics of Extended-Release Oxycodone with Sequestered Naltrexone (ALO-02). Clin Drug Investig 35, 267–274 (2015). https://doi.org/10.1007/s40261-015-0278-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0278-6