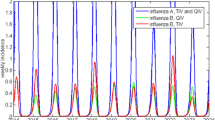
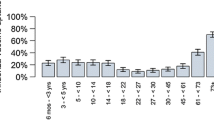
Abstract
Objectives
We estimated the epidemiological and economic impact of extending the French influenza vaccination programme from at-risk/elderly (≥65 years) only to healthy children (2–17 years).
Methods
A deterministic, age-structured, dynamic transmission model was used to simulate the transmission of influenza in the French population, using the current vaccination coverage with trivalent inactivated vaccine (TIV) in at-risk/elderly individuals (current strategy) or gradually extending the vaccination to healthy children (aged 2–17 years) with intranasal, quadrivalent live-attenuated influenza vaccine (QLAIV) from current uptake up to 50% (evaluated strategy). Epidemiological, medical resource use and cost data were taken from international literature and country-specific information. The model was calibrated to the observed numbers of influenza-like illness visits/year. The 10-year number of symptomatic cases of confirmed influenza and direct medical costs (‘all-payer’) were calculated for the 0–17- (direct and indirect effects) and ≥18-year-old (indirect effect). The incremental cost-effectiveness ratio (ICER) was calculated for the total population, using a 4% discount rate/year.
Results
Assuming 2.3 million visits/year and 1960 deaths/year, the model calibration yielded an all-year average basic reproduction number (R 0) of 1.27. In the population aged 0–17 years, QLAIV prevented 865,000 influenza cases/year (58.4%), preventing 10-year direct medical expenses of €374 million. In those aged ≥18 years with unchanged TIV coverage, 1.2 million cases/year were averted (27.6%) via indirect effects (additionally prevented expenses, €457 million). On average, 613 influenza-related deaths were averted annually overall. The ICER was €18,001/life-year gained. The evaluated strategy had a 98% probability of being cost-effective at a €31,000/life-year gained threshold.
Conclusions
The model demonstrated strong direct and indirect benefits of protecting healthy children against influenza with QLAIV on public health and economic outcomes in France.




Similar content being viewed by others
References
Wong KK, et al. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics. 2013;132(5):796–804.
INSERM. Dossier réalisé en collaboration avec Bernadette Murgue, Institut de microbiologie et des maladies infectieuses (Aviesan). Janvier 2012 July 2015]; http://www.inserm.fr/thematiques/immunologie-inflammation-infectiologie-et-microbiologie/dossiers-d-information/grippe. Accessed 25 Nov 2016.
Pitman R, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–5. Value Health. 2012;15(6):828–34.
Weycker D, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–93.
Vynnycky E, et al. Estimating the impact of childhood influenza vaccination programmes in England and Wales. Vaccine. 2008;26(41):5321–30.
Pitman RJ, White LJ, Sculpher M. Estimating the clinical impact of introducing paediatric influenza vaccination in England and Wales. Vaccine. 2012;30(6):1208–24.
Pitman RJ, Nagy LD, Sculpher MJ. Cost-effectiveness of childhood influenza vaccination in England and Wales: results from a dynamic transmission model. Vaccine. 2013;31(6):927–42.
Baguelin M, et al. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527.
Kostova D, et al. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One. 2013;8(6):e66312.
Rose MA, et al. The epidemiological impact of childhood influenza vaccination using live-attenuated influenza vaccine (LAIV) in Germany: predictions of a simulation study. BMC Infect Dis. 2014;14:40.
Damm O, et al. Public health impact and cost-effectiveness of intranasal live attenuated influenza vaccination of children in Germany. Eur J Health Econ. 2015;16(5):471–88.
Beutels, P., et al., Seasonal influenza vaccination: prioritizing children or other target groups? Part II: cost-effectiveness analysis. Health Technology Assessment (HTA) Brussels: Belgian Health Care Knowledge Centre (KCE). KCE Reports 204. 2013.
INSEE. Population—Publications et statistiques pour la France ou les régions. July 2015]; http://www.insee.fr/fr/themes/theme.asp?theme=2. Accessed 25 Nov 2016.
Tuppin P, et al. Influenza vaccination coverage in France in 2007–2008: contribution of vaccination refund data from the general health insurance scheme. Med Mal Infect. 2009;39(10):780–8.
HAS, Choix méthodologiques pour l’évaluation économique à la HAS - Guide méthodologique. Octobre 2011.
Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74.
Carrat F, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–85.
Whitley RJ, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20(2):127–33.
Heikkinen T, et al. Burden of influenza in children in the community. J Infect Dis. 2004;190(8):1369–73.
Tuppin P, et al. Seasonal influenza vaccination coverage in France during two influenza seasons (2007 and 2008) and during a context of pandemic influenza A(H1N1) in 2009. Vaccine. 2011;29(28):4632–7.
Tuppin P, et al. Vaccination against seasonal influenza in France in 2010 and 2011: decrease of coverage rates and associated factors. Presse Med. 2012;41(11):e568–76.
GEIG. (Groupe d’expertise et d’information sur la grippe). Evolution du taux de couverture vaccinale selon l’âge. http://www.grippe-geig.com/couverture-vaccinale.html. Accessed 25 Nov 2016.
Rhorer J, et al. Efficacy of live attenuated influenza vaccine in children: a meta-analysis of nine randomized clinical trials. Vaccine. 2009;27(7):1101–10.
Jefferson T, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;2:CD004879.
Monto AS, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361(13):1260–7.
Jefferson T, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;2:CD004876.
Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J. 2010;29(9):806–11.
Tam JS, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J. 2007;26(7):619–28.
ameli.fr. Assurance Maladie. July 2015]; http://www.ameli.fr/assures/soins-et-remboursements/index.php. Accessed 25 Nov 2016.
ATIH. Agence Technique de l’Information sur l’Hospitalisation. July 2015]; http://www.atih.sante.fr/. Accessed 25 Nov 2016.
INVS, Équipes de surveillance de la grippe. Surveillance épidémiologique et virologique de la grippe en France métropolitaine: saison 2012–2013. Bull Épidemiol Hebd. 2013;32:394–401.
Carrat F, Valleron AJ. Influenza mortality among the elderly in France, 1980–90: how many deaths may have been avoided through vaccination? J Epidemiol Community Health. 1995;49(4):419–25.
Gerlier L, et al. Estimates of the public health impact of a pediatric vaccination program using an intranasal tetravalent live-attenuated influenza vaccine in Belgium. Paediatr Drugs. 2016;18(4):303–18.
Mauskopf JA, et al. Cost effectiveness of zanamivir for the treatment of influenza in a high risk population in Australia. Pharmacoeconomics. 2000;17:611–20.
Glasser J, et al. Evaluation of targeted influenza vaccination strategies via population modeling. PLoS One. 2010;5(9):e12777.
Bonmarin I, Levy-Bruhl D. Analyse des données d’hospitalisation en France à partir du PMSI pendant la période pandémique 2009/2010.
SPILF, Société de Pathologie Infectieuse de Langue Française (SPILF): Prise en charge de la grippe en dehors d’une situation de pandémie en 2005—Texte Long. 2005: Médecine et maladies infectieuses 35. p. S245–S273.
Monto AS, Koopman JS, Longini IM. Tecumseh study of illness. XIII. Influenza infection and disease, 1976-1981. Am J Epidemiol. 1985;121(6):811–22.
Van Kerckhove K, et al. The impact of illness on social networks: implications for transmission and control of influenza. Am J Epidemiol. 2013;178(11):1655–62.
Béraud G, et al. The French connection: the first large population-based contact survey in France relevant for the spread of infectious diseases. PLoS One. 2015;10(7):e0133203.
Vesikari T, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365(15):1406–16.
Carrat F, et al. Influenza burden of illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med. 2002;162(16):1842–8.
Blanchon T. Prescriptions et délivrance des antiviraux en médecine de ville dans le traitement de la grippe pandémique et saisonnière: Rétro-Dina, un travail en commun avec le réseau Sentinelles, in Réseau des GROG: XVIIème Journée Scientifique Nationale. 3 Oct 2013: Paris.
Mosnier A, Cohen J, Daviaud I, Groupes Régionaux d’Observation de la Grippe (GROG). Estimation GROG du coût direct de l’épidémie de grippe 2005/2006, in Journées Nationales d’Infectiologie. 2007: Dijon. p. C02.
Cohen J, et al. Etude du Fardeau de la Grippe chez les enfants de moins de 15 ans consultant en médecine générale ou en pédiatrie pour une infection respiratoire aiguë. Etude EFG Junior. Rapport d’étude. 1—Analyse descriptive. July 2011.
Mosnier A, et al. Open Rome. Etude EFG Senior. Rapport d’étude. July 2012.
Vincent S, et al. Management of influenza-like illness by homeopathic and allopathic general practitioners in France during the 2009–2010 influenza season. J Altern Complement Med. 2013;19(2):146–52.
Pelat C, et al. Hospitalization of influenza-like illness patients recommended by general practitioners in France between 1997 and 2010. Influenza Other Respir Viruses. 2013;7(1):74–84.
Meier CR, et al. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19(11):834–42.
Catherinot E, et al. Service de pneumologie Hôpital Foch. La pneumologie fondée sur les preuves, Chapitre 1. Infections respiratoires basses communautaires. Sous l’égide la SPLF., E.M. Orange, Editor. 21 Octobre 2013.
Acknowledgements
We thank Gilles Berdeaux for his role in the study and experts’ board conduct.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This study was funded by an unrestricted grant from AstraZeneca France.
Conflicts of interest
LG and ML are employees of IMS Health, which received consulting fees from AstraZeneca. SG is employed by AstraZeneca. OD has conducted studies for and received honoraria from Herescon GmbH, which has received research support and consulting fees from AstraZeneca and MedImmune. MS is an employee and shareholder of ExploSYS GmbH, which has received payments from Epimos GmbH, a contract research and consulting institute, which has received research support and consulting fees from AstraZeneca. ME is a partner and shareholder of the contract research and consulting institute Epimos GmbH, which received consulting fees and research support from AstraZeneca, Novartis and GlaxoSmithKline. FC has received consulting fees from AstraZeneca and GlaxoSmithKline. XL has received consulting fees from AstraZeneca. CWO has received grants for congresses and honoraria for conferences and meetings from AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, Sanofi-Pasteur, and Sanofi-Pasteur MSD.
Authorship
ME and OD conceptualised the study, carried out the simulations and interpreted the results. MS designed and developed the simulation tool and provided technical support. LG provided local data input, analysed the simulation results and drafted the manuscript. CWO, FC and XL were part of the scientific committee of the project; they provided expertise and guidance on data input and assumptions. SG provided clinical data inputs and coordinated the discussions with the scientific committee. All authors critically appraised, corrected and validated the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gerlier, L., Lamotte, M., Grenèche, S. et al. Assessment of Public Health and Economic Impact of Intranasal Live-Attenuated Influenza Vaccination of Children in France Using a Dynamic Transmission Model. Appl Health Econ Health Policy 15, 261–276 (2017). https://doi.org/10.1007/s40258-016-0296-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0296-4