Abstract
In current clinical practice, peripherally inserted central catheters (PICCs) are typically inserted using external anatomical measurements and a confirmatory chest X-ray, or using fluoroscopy. The Sherlock 3CG® Tip Confirmation System (TCS) allows magnetic tracking of the PICC tip during insertion and confirmation of the final location using ECG, meaning that most patients will not require a chest X-ray or fluoroscopy. The Sherlock 3CG® TCS was evaluated in 2014 by the UK National Institute for Health and Care Excellence (NICE) as part of the Medical Technologies Evaluation Programme. The company (C.R. Bard Ltd) identified four abstracts, one paper pending publication and questionnaire data from NHS users of the Sherlock 3CG® TCS. None of the evidence included a comparator arm. Placement accuracy of PICCs using the Sherlock 3CG® TCS where a chest X-ray was also used ranged from 79.5 to 100 %. The company reported that 9 out of 16 NHS centres that used the Sherlock 3CG® TCS were no longer using chest X-rays to routinely confirm PICC tip location. The evidence did not report the need for catheter repositioning, re-insertion, staff time savings, treatment delays, length of stay, quality of life outcomes or complications. The company’s model found that the Sherlock 3CG® TCS was cost saving by GBP25.67 per patient compared to blind bedside PICC insertion. The External Assessment Centre (EAC) adapted the company’s model to test alternative assumptions for nurse time, theatre cost, malposition rate and reinsertion method, and found that the Sherlock 3CG® TCS was cost incurring by GBP9.37 per patient compared to blind bedside PICC insertion. The use of the Sherlock 3CG® TCS in the UK NHS compared to blind PICC insertion using a confirmatory chest X-ray appears to hover around being cost neutral. Staff time and accuracy were key drivers in the model: evidence for these is sparse and the reality will vary in different situations. If evidence became available for outcomes after the initial insertion, such as replacement, complications and adverse events, the cost implications may change. The direction of this potential change is not known. NICE published guidance MTG24 in March 2015 recommending that the case for adoption of Sherlock 3CG® TCS was supported by the evidence.
Similar content being viewed by others
The Sherlock 3CG® TCS has already been implemented in 16 NHS sites, and routine use of chest X-rays for confirming PICC tip location has been eliminated in some of these. |
Use of the Sherlock 3CG® TCS appears to be approximately cost neutral compared to blind PICC insertion, based on the available low quality evidence. It may be cost saving if there are reductions in nurse time, X-ray provision, portering or the number of reinsertions required. |
There is some evidence that the Sherlock 3CG® TCS can improve tip positioning accuracy compared to blind placement of PICCs. |
There is no evidence on the effect of Sherlock 3CG® TCS on the time to treatment, length of stay, clinical outcomes or patient experience. |
1 Introduction
This is part of a series summarising guidance produced by the National Institute for Health and Care Excellence (NICE) Medical Technologies Evaluation Programme (MTEP). The process is explained by Campbell and Campbell in the first publication of this series [1]. This article summarises the External Assessment Centre (EAC) report [2] and Medical Technology Guidance (MTG) for the Sherlock 3CG® Tip Confirmation System (TCS) for placement of peripherally inserted central catheters [3]. The EAC that produced the assessment report for the Sherlock 3CG® TCS was Cedar, a collaboration between Cardiff and Vale University Health Board, Cardiff University and Swansea University.
2 Background
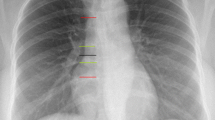
Peripherally inserted central catheters (PICCs) use a catheter inserted at a peripheral vein using ultrasound guidance and gently moved through the vein until the tip is in the superior vena cava or right atrium. The insertion site is typically the brachial or cephalic vein in the upper arm, leading to the right subclavian vein [4, 5] (Fig. 1).
PICCs are used in different circumstances, including:
-
measuring circulatory or heart functions
-
providing long-term access route for infusions and blood tests
-
delivering drugs that require rapid dilution, e.g. chemotherapy
-
delivering contrast medium for cardiac imaging.
The different clinical uses for PICCs mean that there is variation in the pathways that occur in practice. PICCs may be inserted in intensive care units (ICUs), in outpatient clinics, at the bedside, and in fluoroscopy and X-ray departments. The clinical staff who insert PICCs include specialist nurses, radiologists and doctors [2].
The two methods for placing PICCs which are currently standard are [2]:
-
Blind placement—the catheter is pushed into the vein a set distance according to anatomical measurements taken externally on the patient. Once the PICC is placed, the correct position is confirmed using a chest X-ray.
-
Fluoroscopy-guided PICC placement—the tip location can be visualised and the final location confirmed during the procedure.
In the UK, the preferred position for the PICC tip position is normally the mid or lower superior vena cava, cavo-atrial junction, or the high right atrium, based on European guidelines [4]. There is no international consensus.
The length of time for which PICCs are in place ranges from a few days to over a year: 1 week to 3 months is most typical. During this time, the location of the tip may move slightly. This would be identified only if it caused a clinical problem, or if there was an X-ray or imaging for another reason.
There are instances of gross malpositioning, for example if the catheter enters the left subclavian vein or the right internal jugular vein. It may be possible to correct such malpositioning with the catheter in place. Otherwise, the PICC is removed and a new PICC inserted. PICC malpositioning may cause complications such as catheter malfunction, cardiac arrhythmia or tamponade [5]. More minor malpositioning has less obvious clinical relevance. The confirmatory chest X-ray may show that the tip is inserted slightly too far, and in this case it can be pulled back to achieve the desired position. Some PICC teams will intend to always place the tip too far, and pull back by a measured amount following the chest X-ray. The PICC can never be moved further in after initial insertion, as the external section is no longer sterile.
2.1 Sherlock 3CG® Tip Confirmation System
The Sherlock 3CG® TCS uses a sensor to track a magnetic tip at the distal end of the catheter and internal ECG measurements to confirm the position of the catheter tip relative to the heart. For most patients, a chest X-ray will not be required to confirm tip placement, and the procedure can be completed at the patient’s bedside, or in an outpatient clinic.
The Sherlock 3CG® TCS sensor is a class I medical device, which obtained a CE mark in December 2011. The sensor must be used with the Sherlock 3CG® TCS magnetic stylet, and Power PICC SOLO catheter—class II medical devices, which obtained CE marking in February 2012. The Sherlock 3CG® TCS cannot be used with other types of catheter.
2.2 National Institute for Health and Care Excellence (NICE) scope
NICE defines the scope of the evaluation prior to the company’s submission of evidence [6].
Population Adult patients undergoing PICC insertion.
Intervention The Sherlock 3CG® TCS, used by a healthcare professional trained in PICC placement. Previous versions of the system that did not include the use of both magnetic tracking and ECG tip confirmation were excluded.
Comparators There were two comparators, based on current practice:
-
Blind PICC insertion followed by chest X-ray.
-
Fluoroscopy.
Outcomes Included accuracy of catheter tip placement, requirement for confirmatory chest X-ray, catheter malposition rates, catheter re-positioning rates, impact of malposition-related complications, time to treatment, staff time, length of stay, fluoroscopy needed to place the PICC, time for PICC insertion, patient experience, patient quality of life and device-related adverse events.
The benefits to patients claimed by the company are [2, 6, 7]:
-
Better accuracy of PICC placement.
-
Better outcomes by reducing the incidence of catheter malposition and post-procedural repositioning.
-
Removed the need for a chest X-ray or fluoroscopy to confirm tip location after PICC insertion.
-
Reduced treatment delays due to intra-procedural verification of tip position.
-
Safe method for PICC tip placement with no associated adverse events or complications.
-
PICC placement and tip verification are during the same procedure.
-
It improves patient experience and increases the patient’s confidence in the PICC placer.
The benefits to the healthcare system claimed by the company are:
-
A reduced and more efficient care pathway because no chest X-ray is needed.
-
Lower staff requirements (radiologists/radiology nurses/radiographers/radiology healthcare support workers) because the need for X-ray confirmation is reduced. Also reduced need for porters for patient transfer and doctors for X-ray assessment.
-
Potential reduction of bed occupancy due to reductions in treatment delays.
-
Reduced costs of consequences of malpositioning.
-
Reduced costs of using resource intensive departments such as radiology.
3 Review of Clinical and Economic Evidence
The company provided an evidence submission to NICE that summarised the clinical and cost evidence for the Sherlock 3CG® TCS and presented a cost-consequence model [8]. The aim is to evaluate whether the Sherlock 3CG® TCS carries a clinical advantage and/or a reduced cost in comparison to current NHS standard care.
3.1 Company’s submission of clinical effectiveness evidence
The company identified and submitted 4 relevant studies [9–12]. None of the submitted studies were peer reviewed or published in full. Two studies were from the USA, one from Australia and one from the UK. The company also identified a study that was pending publication [13], and subsequently published. Given the paucity of evidence, the EAC widened the scope to include any previous models of the technology that included both ECG measurement and magnetic tracking. This resulted in one presentation being included [14]. The company also submitted information from questionnaires that it had sent to selected NHS users of the Sherlock 3CG® TCS.
3.2 Critique of clinical evidence available
3.2.1 Peer reviewed evidence
The only available peer reviewed paper reported a retrospective analysis of the first 250 PICC insertions following introduction of the Sherlock 3CG® TCS to an NHS intensive care unit (ICU) [13]. PICCs were placed by the Vascular Access Team at the bedside and, following tip confirmation with the Sherlock 3CG® TCS, a portable X-ray was used to assess placement accuracy. Eleven out of 250 patients (4 %) were excluded due to difficulties interpreting the ECG (n = 4), other PICC difficulties (n = 3), or X-ray difficulties (n = 4). The authors reported 49 out of 239 (21 %) tip placements were not in the correct position using European guidelines (Pittiruti guideline).
A paper by the same group reported accuracy of blind bedside PICC placement shortly before the introduction of the Sherlock 3CG® TCS [15]. The EAC has presented the results of both papers in Table 1, although the studies do not directly compare identical populations. The malposition rate using the Sherlock 3CG® TCS is significantly lower than the blind placement (p < 0.0001) and the authors conclude that if this definition of tip placement is acceptable, then the Sherlock 3CG® TCS can be used for tip confirmation without routine chest X-ray confirmation.
3.2.2 Other Evidence
Results from non-peer-reviewed evidence such as abstracts, posters and presentations are summarised in Table 2.
The accuracy of the Sherlock 3CG® TCS in Johnston et al. [13] (Table 1) is lower than that reported in five other studies (Table 2); possibly because ICU patients have less clear heart rhythm or are recumbent. It is unclear if the results from this one study are generalisable to other settings (either to other ICU settings or the wider NHS).
Questionnaires completed by six NHS sites using the Sherlock 3CG® TCS highlighted the variation in normal clinical pathways for tip insertion and confirmation, repositioning and reinsertion. At the time of submission, the company reported that nine NHS sites had stopped using routine chest X-rays for tip confirmation, from a total of 16 NHS sites that have introduced the Sherlock 3CG® TCS.
3.2.3 Summary
All studies used the Sherlock 3CG® TCS as the intervention; none were comparative. The studies reported the use of the Sherlock 3CG® TCS for tip confirmation, followed by a chest X-ray to determine if the tip was in the correct place. There were no comparators giving information on the blind PICC placement malposition rates in the same setting. The studies all stop at the point of tip confirmation and assessment by chest X-ray. It is not known if PICCs identified as malpositioned had to be adjusted or re-inserted, or what the consequences might be. Similarly, the studies do not give any indication if any patients would have been identified as having misplaced PICCs without the chest X-ray, and what the clinical implications might have been.
Several of the sites from the published abstracts [9–12, 14] report that routine chest X-rays are no longer used to confirm PICC placement. The sponsors report that nine NHS sites using Sherlock 3CG® TCS have had sufficient confidence in the device to remove the requirement for routine chest X-rays to confirm tip location.
3.3 Cost Evidence
Two studies included cost information [9, 10], but contained insufficient information for critical analysis, and had limited relevance to the decision problem. The EAC did not find any additional studies that should have been included. Only two of the six clinical studies identified report the time taken to insert the PICC and complete tip confirmation using Sherlock 3CG® TCS (without chest X-ray), and compare it to the time taken by the previous method. There was no evidence for the effect on staff time, time to deliver treatment, bed days, clinical outcome or patient experience.
The company created an economic model with four branches:
-
1.
Bedside PICC placement with Sherlock 3CG® TCS with X-ray.
-
2.
Bedside PICC placement with Sherlock 3CG® TCS without X-ray.
-
3.
Blind bedside PICC placement, with X-ray.
-
4.
Fluoroscopy guided placement.
Inputs to the model were based on the studies and NHS questionnaires submitted as clinical evidence. Staff costs were derived from the Personal Social Services Research Unit (PSSRU) [16], and detailed pathway information and costs for blind bedside PICC placement and fluoroscopy were obtained from Walker et al. [17, 18].
Using this cost model, the company found that the Sherlock 3CG® TCS without X-ray gave a cost saving of GBP25.67 per patient compared to blind bedside PICC insertion, and a cost saving of GBP510.03 per patient compared to fluoroscopy. All costs are based on 2014 values.
3.4 Critique of Economic Submission
There are uncertainties in the model structure and inputs due to the lack of data available and the variations in patient groups and service provision. The company carried out extensive sensitivity analysis, although the EAC disagreed with some of the key inputs. Important assumptions and data for the model submitted by the company, along with the views of the EAC, are listed below.
3.4.1 Time Horizon
The model stops at the point of confirming PICC tip placement. The EAC considered that a preferable time horizon would include adverse events, complications, the time to starting treatment and the clinical consequences. Unfortunately, there is no evidence available to inform such a model and the impact and direction of any effect is unknown.
3.4.2 Nurse Time
The difference in staff time is one of the model’s key drivers. The two studies used in the company’s model were from different countries with different health systems, causing the EAC to consider comparability unlikely. The values used by the company were:
-
62.49 min for blind PICC insertion with X-ray and the Sherlock 3CG® TCS with X-ray, based on Walker and Todd [17] bedside placement time.
-
39.5 min for the Sherlock 3CG® TCS without X-ray [9].
3.4.3 Reinsertion Rates and Method
The difference in reinsertion rates and cost of reinsertion is a key driver when comparing blind bedside PICC insertion to the Sherlock 3CG® TCS. The proportion of accurate placement the model uses is:
-
96 % for the Sherlock 3CG® TCS [10] for trained staff. This study used a comparison to X-ray to judge accuracy. Where the normal pathway does not include a chest X-ray, a malposition will only be detected if it causes a clinical problem.
-
93.1 % for blind bedside PICC placement [17].
-
100 % for fluoroscopy [17].
The base case of the cost model assumes that all identified tip malpositions will require replacement, and that all reinsertions would be performed using fluoroscopy. Not all malpositions will require reinsertion procedures, and although some services may reinsert PICCs using fluoroscopy, others will use bedside techniques. Because the model includes more malpositions for blind PICC placement than for the Sherlock 3CG® TCS, and fluoroscopy is the most costly option, these assumptions favour the Sherlock 3CG® TCS.
3.4.4 Patient Population
The scope defined by NICE included all adult patients undergoing PICC insertion. The population included in the company’s model was adult patients undergoing PICC insertion who were suitable for ECG tip confirmation using the Sherlock 3CG® TCS. The company assumed 83.5 % of patients were suitable [9], and did not include costs for treatment of the remaining 16.5 % by an alternative method. This has a low impact on the model where the two comparative arms have similar costs. Expert advice indicated that the proportion of patients for whom the Sherlock 3CG® TCS is suitable was likely to be higher than 83.5 %. The company’s instructions for use state that use of the technology is limited (but not contraindicated) in patients who do not have an identifiable P wave, e.g. those with atrial fibrillation, tachycardia, etc.
3.4.5 Fluoroscopy
The company’s model finds a large cost saving using the Sherlock 3CG® TCS at the bedside compared to fluoroscopy. This is partially due to the high cost attributed to fluoroscopy—much of the remaining cost saving is due to the move to bedside placement and would be equally true of blind PICC bedside placement.
The cost of theatre for fluoroscopy was assumed by the company to be GBP507.18 [17, 18]. The EAC estimated this cost to be GBP101 [19].
If standard practice was fluoroscopy, and a new bedside PICC placement service had to be set up, there would be costs for nurse training and reorganisation. The model does not include these initial set up costs.
3.5 EAC Alternative Scenarios
There are unavoidable uncertainties due to the lack of evidence, and the variations in patient groups and service provision. There are many inputs and scenarios that can change the model from being cost saving to cost incurring—the most accurate representation will depend on the service being examined. The EAC analysis presents an alternative set of assumptions—there is insufficient evidence to allow absolute certainty over which is most appropriate.
3.5.1 EAC Changes to Company’s Model
The EAC changes to the company’s base model were:
-
Nurse time is equal for blind PICC insertion with X-ray, the Sherlock 3CG® TCS with X-ray and the Sherlock 3CG® TCS without X-ray. The EAC used a time of 62.49 min [17]. X-rays are costed separately.
-
Where the Sherlock 3CG® TCS is used without X-ray, there is an assumption of zero malpositions being identified within the time frame of the model, since no other tip confirmation system is used.
-
Replacements for PICCs placed at the bedside are by the same method as the original placement.
-
In patients for whom use of the Sherlock 3CG® TCS is not suitable, treatment costs by an alternative method were included.
-
The cost of theatre use for fluoroscopy was changed to GBP101 [19].
Using these inputs, the EAC found that the Sherlock 3CG® TCS without X-ray incurred a cost of GBP9.37 per patient compared to blind bedside PICC insertion, and gave a cost saving of GBP106.12 per patient compared to fluoroscopy. All costs are based on 2014 values.
3.5.2 EAC Cost Neutral Scenario
The EAC explored a scenario where the use of the Sherlock 3CG® TCS is approximately cost neutral when the following assumptions are made (in addition to the previous EAC changes):
-
Nurse time for the Sherlock 3CG® TCS is 57.5 min, a 5-min reduction from the original EAC assumption, as there is no requirement for the nurse to interpret the X-ray image.
-
The cost of X-ray is assumed to be 10 min of radiologist time (GBP5.67, company’s submission, based on Walker and Todd [17]) with the addition of 15 min for a Band 2 porter (GBP5.25 [16]) to transport patient to and from X-ray. This gives total X-ray cost of GBP10.92.
Using these inputs, the EAC found that the Sherlock 3CG® TCS without X-ray was associated with a cost saving of GBP1.17 per patient compared to blind bedside PICC insertion, and a cost saving of GBP111.27 per patient compared to fluoroscopy.
3.5.3 EAC Scenario Based on ICU Data
The EAC also explored a scenario based on ICU data [13, 15], changing the accuracy rates to those in Table 1. Sherlock 3CG® TCS with X-ray was used in the model, reflecting the local practice at the time of publication [13]. The limitations of this scenario are:
-
the data is taken from a single centre and may not be generalisable.
-
the comparison is with historical data from the same centre.
-
the study only includes intermediate outcomes—the actual number of replacements is not reported.
In this scenario, the Sherlock 3CG® TCS with X-ray confirmation is associated with a cost saving of GBP41.35 per patient compared to blind bedside PICC insertion, because of the significant reduction in the proportion of malpositioned catheters that needed to be re-positioned.
3.6 Impact of Changes
Given current information, use of the Sherlock 3CG® TCS compared to blind PICC insertion using a chest X-ray appears to hover around cost neutral. Staff time and accuracy are key drivers in the model, but evidence for these is sparse and the reality will vary in different situations. If evidence became available for outcomes after the initial insertion, such as replacement, complications and adverse events, the cost implications may change. The direction of this potential change is not known.
4 NICE Guidance
4.1 Preliminary Guidance
The NICE Medical Technologies Advisory Committee (MTAC) met in October 2014 and considered evidence from a range of sources, including the company’s submission, the EAC report and additional economic modelling, and testimony from clinical experts. The committee made provisional recommendations that went to public consultation.
The Committee considered the overall quality and quantity of the evidence to be low, but noted that the general trend of the clinical evidence was in favour of the Sherlock 3CG® TCS, and that the technology seemed likely to be cost neutral. Some additional analysis was carried out by the EAC to further test the impact of staff time on the cost model.
4.2 Consultation Response
During the consultation period, NICE received 14 consultation comments from 7 consultees (3 NHS professionals, 2 manufacturers, the Department of Health, and one EAC representative). The majority of these comments were process-related, and considered if the single-technology assessment methods used by MTEP were appropriate for medical technologies evaluation. At the final guidance meeting (January 2015), the Committee considered these comments with reference to the MTEP’s process and methods guides [20, 21] and concluded that they should not impact on the provisional recommendations. Accordingly, the recommendations did not change substantively as a result of public consultation.
4.3 Final Recommendations
The NICE Medical Technology Guidance on the Sherlock 3CG® TCS for placement of peripherally inserted central catheters was published on 25 March 2015 as MTG 24 [3]. It contains the following recommendations:
-
1.
The case for adopting the Sherlock 3CG® TCS for placement of peripherally inserted central catheters is supported by the evidence. The technology usually avoids the need for a confirmatory chest X-ray in patients who would otherwise have blind insertion, minimising the delay before the catheter can be used for infusion. Using the technology increases staff confidence during catheter insertion.
-
2.
The Sherlock 3CG® TCS should be considered as an option for placement of peripherally inserted central catheters in adults. For patients whose electrocardiogram does not show a P wave (for example, patients with atrial fibrillation), a chest X-ray will still be needed to confirm tip location of the peripherally inserted central catheter.
-
3.
The cost of using the Sherlock 3CG® TCS is similar to that of blind insertion and subsequent chest X-ray in adults who need a peripherally inserted central catheter in a non-intensive care setting. When the Sherlock 3CG® TCS is used instead of fluoroscopy, the estimated cost saving is GBP106 per patient. In an intensive care setting, where the rate of misplacement with blind insertion is generally higher, there is an estimated cost saving of GBP41 per patient per use of the Sherlock 3CG® TCS and a confirmatory chest X-ray compared with using blind insertion and chest X-ray. All these cost savings are subject to some uncertainty and need to be considered in the context of the clinical benefits.
5 Key Challenges and Learning Points
The wide range of uses for PICCs meant that it was difficult to identify a typical patient pathway. Clinical expertise was the key to understanding different pathways and their implications.
The most widely reported outcome was accuracy of the PICC placement judged against chest X-ray. This is an intermediate outcome, rather than the actual clinical or economic information important to a decision.
Sensitivity analysis and alternative scenarios helped to understand the range of possible outcomes within the structure of the model, and to reflect the diverse clinical realities. Where there is no clinical evidence for the actual outcomes needed, there can only be limited confidence in the economic model.
References
Campbell B, Campbell M. NICE medical technologies guidance: a novel and rigorous methodology to address a new health technology assessment challenge. Appl Health Econ Health Policy. 2012;10(5):295–7.
Dale M, Morgan H, Peirce S, et al. External Assessment Centre Report: Sherlock 3CG® Tip Confirmation System for placement of peripherally inserted central catheters. 2014. http://www.nice.org.uk/guidance/mtg24/documents. Accessed 18 June 2015.
National Institute of Health and Care Excellence. Sherlock 3CG® Tip Confirmation System for placement of peripherally inserted central catheters MTG24. 2015. https://www.nice.org.uk/guidance/mtg24. Accessed 2 Apr 2015.
Pittiruti M, Hamilton H, Biffi R, et al. ESPEN Guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009;28(4):365–77.
Amerasekera S, Jones C, Patel R, et al. Imaging of the complications of peripherally inserted central venous catheters. Clin Radiol. 2009;64:832–40.
National Institute of Health and Care Excellence. Scope: Sherlock 3CG® Tip Confirmation System for placement of peripherally inserted central catheters MTG24. 2014. http://www.nice.org.uk/guidance/mtg24/documents. Accessed 18 June 2015.
National Institute of Health and Care Excellence. Assessment Report Overview: Sherlock 3CG® Tip Confirmation System for placement of peripherally inserted central catheters MTG24. 2014. http://www.nice.org.uk/guidance/mtg24/documents. Accessed 18 June 2015.
C.B Bard. Sponsor submission: Sherlock 3CG® Tip Confirmation System for placement of peripherally inserted central catheters MTG 2014. 2015. http://www.nice.org.uk/guidance/mtg24/documents. Accessed 18 June 2015.
Adams T. The clinical efficacy of PICC tip confirmation using ECG tip locator technology [abstract no. 83 plus poster]. Association of vascular access annual scientific Meeting, Sep 20–23 Nashville; 2013. http://www.eventscribe.com/2013/posters/ava/SplitViewer.asp?PID=MjA3ODQ5ODg5. Accessed 2 Apr 2015.
Parikh A, Babcock L, Brumble L, et al. Successful implementation of electrocardiocardiographic-guided picc placement with a nurse-comprised PICC Team. (Poster) Radiological Society of North America; 2012.
Stewart F. New Australian Experience: Improvement in Peripherally Inserted Central Catheter (PICC) Insertion technique using real time tracking and ECG for optimal PICC tip position (without need for CXR). (abstract 39 plus poster) Association of Vascular Access Annual Scientific Meeting, Sep 20–23 Nashville; 2013. http://www.eventscribe.com/2013/posters/ava/SplitViewer.asp?PID=MjA2MzgxOTU2. Accessed 2 Apr 2015.
Barton A. Evaluation of ECG and PICC tip location technology; is it safe to stop using X-ray. J Vasc Access. 2014;15(3)S7: 202 (Abstract plus oral presentation) World Congress of Vascular Access.
Johnston A, Holder A, Bishop SM, et al. Evaluation of the Sherlock 3CG® Tip Confirmation System on peripherally inserted central catheter malposition rates. Anaesthesia. 2014;69(12):1322–30.
Symington K. The Spokane Sapiens TCS experience…are you next (Oral presentation), association of vascular access: San Jose; 2011.
Johnston A, Bishop SM, Martin L, et al. Defining peripherally inserted central catheter tip position and an evaluation of insertions in one unit. Anaesthesia. 2014;2013(5):484–91.
Curtis L. Unit costs of health and social care 2012, PSSRU. 2012.
Walker G, Todd A. Nurse-led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–15.
Walker G. Investigation of the health economics of peripherally inserted central catheter (PICC) placement by different staff groups in a district general hospital (dissertation). Aberdeen: University of Aberdeen; 2013.
Department of Health. NHS Reference Costs, Financial year 2012–13.
NICE. Medical Technologies Evaluation Programme: methods guide. 2011. Available from https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-medical-technologies-guidance. Accessed 2 Apr 2015.
NICE. Medical Technologies Evaluation Programme: process guide. 2011. Available from https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-medical-technologies-guidance. Accessed 2 Apr 2015.
Acknowledgments
The authors thank Dr. Helen Morgan (Cedar), Andrew Cleves (Cedar), Dr. Susan Peirce (Cedar)and Pippa Anderson (Swansea Centre for Health Economics, Swansea University) for their contribution to the original EAC report for NICE, Dr. Judith White (Cedar) for reviewing the manuscript, and Jan Sharp (Cardiff University) for the illustration.
Author contributions
MD evaluated the clinical evidence, and both MD and GCR evaluated the economic evidence on which this manuscript is based, as well as the EAC scenarios. MD and GCR wrote the assessment report and AH wrote the assessment report overview both of which were presented to the Medical Technologies Advisory Committee. AH subsequently helped to develop the medical technologies guidance for NICE. GCR reviewed the full EAC report and this article, and can act as a guarantor for the overall content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Cedar is funded by the NICE Medical Technologies Evaluation Programme to act as an EAC. MD and GCR are NHS employees, and the NHS has a financial interest in the guidance on which this project is based. AH works for the National Institute for Health and Care Excellence, and has no conflicts of interests to declare. This article has not been externally peer reviewed by Applied Health Economics and Health Policy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dale, M., Higgins, A. & Carolan-Rees, G. Sherlock 3CG® Tip Confirmation System for Placement of Peripherally Inserted Central Catheters: A NICE Medical Technology Guidance. Appl Health Econ Health Policy 14, 41–49 (2016). https://doi.org/10.1007/s40258-015-0192-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-015-0192-3