Abstract
Background
The recent approval of two protease inhibitors, boceprevir and telaprevir, is likely to change the management of chronic hepatitis C virus (HCV) genotype 1 infection.
Objectives
We evaluated the long-term clinical outcomes and the cost effectiveness of therapeutic strategies using boceprevir with peginterferon plus ribavirin (PR) in comparison with PR alone for treating HCV genotype 1 infection in Portugal.
Methods
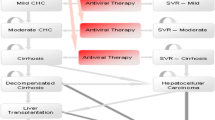
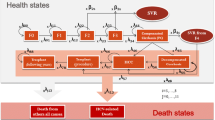
A Markov model was developed to project the expected lifetime costs and quality-adjusted life-years (QALYs) associated with PR alone and the treatment strategies outlined by the European Medicines Agency in the boceprevir summary of product characteristics. The boceprevir-based therapeutic strategies differ according to whether or not the patient was previously treated and whether or not the patient had compensated cirrhosis. The model simulated the experience of a series of cohorts of chronically HCV-infected patients (each defined by age, sex, race and fibrosis score). All treatment-related inputs were obtained from boceprevir clinical trials – SPRINT-2, RESPOND-2 and PROVIDE. Estimates of the natural history parameters and health state utilities were based on published studies. Portugal-specific annual direct costs of HCV health states were estimated by convening a panel of experts to derive health state resource use and multiplying the results by national unit costs. The model was developed from a healthcare system perspective with a timeframe corresponding to the remaining duration of the patients’ lifetimes. Both future costs and QALYs were discounted at 5 %. To test the robustness of the conclusions, we conducted deterministic and probabilistic sensitivity analyses.
Results
In comparison with the treatment with PR alone, boceprevir-based regimens were projected to reduce the lifetime incidence of advanced liver disease, liver transplantation, and liver-related death by 45–51 % and increase life expectancy by 2.3–4.3 years. Although the addition of BOC increased treatment costs by €13,300–€19,700, the reduction of disease burden resulted in a decrease of €5,400–€9,000 in discounted health state costs and an increase of 0.68–1.23 in discounted QALYs per patient. The incremental cost-effectiveness ratios of the boceprevir-based regimens compared with PR among previously untreated and previously treated patients were €11,600/QALY and €8,700/QALY, respectively. The results were most sensitive to variations in sustained virologic response rates, discount rates and age at treatment.
Conclusions
Adding boceprevir to PR was projected to reduce the number of liver complications and liver-related deaths, and to be cost effective in treating both previously untreated and treated patients.




Similar content being viewed by others
References
World Health Organization. Hepatitis C. Fact sheet N°164, June 2011. http://www.who.int/mediacentre/factsheets/fs164/en/index.html. [Accessed 27 Mar 2012].
Marinho R, Giria J, Ferrinho P, Moura MC. Epidemiological aspects of hepatitis C in Portugal. J Gastroenterol Hepatol. 2001;16:1076–7.
Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–73.
Cornberg M, Razavi HA, Alberti A, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl. 2):30–60.
Direcção Geral de Saúde. Incidência da Hepatite C. In: Plano Nacional de Saúde 2004/2010. Vol I – Prioridades. Ministério da Saúde 2011. http://www.dgsaude.min-saude.pt/pns/vol2_223.html [Accessed 2012 Dec 12].
Seeff LB. Natural history of hepatitis C. Hepatology Rev. 2005;2:88–96.
Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71.
Vieira AM, Freire R, Mangualde J, et al. Hepatite C: casuística da consulta de hepatologia de um hospital distrital. GE - J Port Gastrenterol. 2007;14:134–40.
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N England J Med. 2002;347(13):975–82.
Hadziyannis SJ, Sette H Jr, Morgan TR, et al. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–55.
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65.
Asselah T, Marcellin P. New direct-acting antivirals’ combination for the treatment of chronic hepatitis C. Liver Int. 2011;31(1):68–77.
Poordad F, McCone J Jr, Bacon BR, SPRINT-2 Investigators, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
Bacon BR, Gordon SC, Lawitz E, HCV RESPOND-2 Investigators, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–17.
Jensen DM. A new era of HCV therapy begins. N Engl J Med. 2011;364:1272–4.
Ferrante SA, Chhatwal J, Elbasha E, Dasbach E, Bronowicki J-P, Poordad F, et al. Cost-effectiveness of boceprevir based regimens in previously untreated adult subjects with chronic hepatitis C genotype 1. Hepatology. 2011;54(S1):795A–6A.
Chhatwal J, Ferrante SA, Dasbach E, El Khoury A, Brass C, Burroughs M, et al. Cost-effectiveness of boceprevir use in patients with chronic hepatitis C genotype-1 who failed prior treatment with peginterferon/ribavirin. Hepatology. 2012;54(S1):801A–2A.
Buti M, Medina M, Casado MA, Wong JB, Fosbrook L, Esteban R. A cost-effectiveness analysis of peginterferon alfa-2b plus ribavirin for the treatment of naive patients with chronic hepatitis C. Aliment Pharmacol Ther. 2003;17(5):687–94.
Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290(2):228–37.
Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, German Hepatitis C Model (GEHMO) Group; International Hepatitis Interventional Therapy (IHIT) Group, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425–32.
Sullivan SD, Craxi A, Alberti A, Giuliani G, De Carli C, Wintfeld N, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics. 2004;22(4):257–65.
Younossi ZM, Singer ME, McHutchison JG, Shermock KM. Cost effectiveness of interferon 2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology. 1999;30(5):1318–24.
Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–7.
European Medicines Agency. Annex I. summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002332/WC500109786.pdf. [Accessed 27 Mar 2012].
Dieterich DT, Rizzetto M, Manns MP. Management of chronic HCV: definition of relapse and nonresponse. J Viral Hepat. 2009;16(12):833–43.
Velosa J, Serejo F, Bana T, Redondo I, Simão A, et al. Chronic hepatitis C treated with peginterferon alfa plus ribavirin in clinical practice. Hepatogastroenterology. 2011;58(109):1260–6.
Areias J, Gomes H, Mocho ML, et al. Epidemiological characterization of chronic hepatitis C in Portugal. XXI Annual Congress of the “Fundacion y Associacion Espanola para el estudio del higado”, 15–17 Feb 2006; Madrid, Spain.
Vierling JM, Flamm SL, Gordon SC, Lawitz E, Bronowicki J, Davis M, et al. Efficacy of boceprevir in prior null responders to peginterferon/ribavirin: the PROVIDE study. Hepatology. 2011;54(Suppl):796A.
Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618–28.
Instituto Nacional de Estatistica, Statistics Portugal. Portuguese Life Table 2007–2009. http://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=83112819&DESTAQUESmodo=2 [Accessed 1 Jun 2010].
Infarmed, Prontuário Terapêutico On-Line. 2011. http://www.infarmed.pt/prontuario/index.php [Accessed 27 Mar 2012].
Ministério da Saúde, Diário da República—I Série, n°16 (Portaria n° 110-A/2011) de 23 de Janeiro de 2011.
ACSS –online catalogue. http://www.catalogo.min-saude.pt/caps/publico/pub_consulta.asp [Accessed 27 Mar 2012].
Data on file, MSD Portugal, 2010.
INFARMED. Guidelines for Economic Drug Evaluation Studies. Nov 1998. Published in Despacho n° 19064/99; 9 Sep 1999.
Weinstein MC, Toy EL, Sandberg EA, et al. Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001;4(5):348–61.
Wright M, Grieve R, Roberts J, Main J, Thomas HC; UK Mild Hepatitis C Trial Investigators. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10:1–113, iii.
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11:iii–120.
Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–87.
El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and nonliver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–7.
Butt AA, Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin Infect Dis. 2012;54(1):96–104.
World Health Organization. Macroeconomics and health: investing in health for economic development: Report for the Commission on Macroeconomics and Health, Geneva, Switzerland, 2001.
Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, Mühlberger N, Wright D, et al. Long-term effectiveness and cost-effectiveness of antiviral treatment in hepatitis C. J Viral Hepat. 2010;17(1):34–50.
Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279–90.
Cammà C, Petta S, Enea M, Bruno R, Bronte F, Capursi V, et al.; on behalf of the WEF Study Group. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology 2012 Mar 27. doi:10.1002/hep.25734 [Epub ahead of print].
Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–44.
Pearlman B, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900.
Butt AA, Wang X, Moore CM. Effect of HCV and its treatment upon survival. Hepatology. 2009;50:387–92.
Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2001;15:689–98.
Rothwell PM. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–86.
Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31.
Benvegnù L, Noventa F, Bernardinello E, Pontisso P, Gatta A, Alberti A. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut. 2001;48(1):110–5.
Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72.
Gentilini P, Laffi G, La Villa G, Romanelli RG, Buzzelli G, Casini-Raggi V, et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92(1):66–72.
Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17 year cohort study of 214 patients. Hepatology. 2006;43:1303–10.
Serfaty L, Aumaître H, Chazouillères O, Bonnand AM, Rosmorduc O, Poupon RE, Poupon R. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435–40.
Bruno S, Silini E, Crosignani A, Borzio F, Leandro G, Bono F, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology. 1997;25:754–8.
Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328(25):1797–801.
Tateyama M, Yatsuhashi H, Taura N, Motoyoshi Y, Nagaoka S, Yanagi K, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46(1):92–100.
Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131(3):174–81.
Planas R, Ballesté B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis: a study of 200 patients. J Hepatol. 2004;40(5):823–30.
Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1003–19.
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–21.
Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50(1):89–99.
Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16(6):748–59.
Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):961–72.
ACSS – Catálogo. Disponível em: http://www.catalogo.min-saude.pt/caps/publico/pub_consulta.asp [Accessed 27 Mar 2012].
Data on file, Merck Sharp & Dohme (MSD).
Base de Dados do INFARMED – Infomed. http://www.infarmed.pt/infomed/inicio.php [Accessed 27 Mar 2012].
Portaria n.º 132/2009, de 30 de Janeiro - Alterada pela Portaria n.º 839 -A/2009, de 31 de Julho, e pela Portaria n.º 19/2012, de 20 de Janeiro. http://www.portaldasaude.pt/NR/rdonlyres/7A929657-9E9D-4FCC-85E1-A5DC40E64414/0/Portaria132_2009Regulamentopre%C3%A7osSNS.pdf [Accessed 27 Mar 2012].
Acknowledgments
The authors would like to thank Drs John R. Cook and Erik J. Dasbach (Merck) for providing helpful suggestions during model development and Jane Liao (Merck) for providing programming support in the analysis of data from SPRINT-2 and RESPOND-2 studies. The authors would also like to acknowledge the contribution of a panel of eight anonymous Portuguese clinical experts in estimating resource use. All panel members received compensation for their participation, and some members had received speaker’s and investigator’s fees from MSD Portugal in the past.
Disclosures
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. Dr. Chhatwal was a former employee of and has received consulting fees from Merck. Dr. El Khoury was a former employee and Drs Elbasha, Ferrante and Laires are current employees of Merck and all hold stocks and/or stock options.
Author contributions
E.H. Elbasha, J. Chhatwal, S.A. Ferrante and A.C. El Khoury developed the model. P.A. Laires contributed to data collection and analysis. All authors provided critical input to the draft. All authors reviewed the final draft and agree with its content. E.H. Elbasha is a guarantor for the overall content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elbasha, E.H., Chhatwal, J., Ferrante, S.A. et al. Cost-Effectiveness Analysis of Boceprevir for the Treatment of Chronic Hepatitis C Virus Genotype 1 Infection in Portugal. Appl Health Econ Health Policy 11, 65–78 (2013). https://doi.org/10.1007/s40258-012-0007-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-012-0007-8