Abstract
Background
This study was to evaluate the efficacy and safety of triple fixed-dose combination (FDC) therapy with olmesartan medoxomil (OM) 20 mg, amlodipine (AML) 5 mg, and hydrochlorothiazide (HCTZ) 12.5 mg (OM/AML/HCTZ 20/5/12.5) in Korean patients with moderate hypertension not controlled with dual FDC therapy (OM/HCTZ 20/12.5).
Methods
In this multicenter, randomized, double-blind, parallel-group study, Korean patients aged 20 to 75 years with stage 2 hypertension who had a mean seated diastolic blood pressure (msDBP) ≥100 mmHg were enrolled when their BP was uncontrolled [mean seated systolic BP (msSBP)/msDBP >140/90 mmHg or msSBP/msDBP >130/80 mmHg with diabetes or chronic kidney disease] with 4-week dual FDC therapy (OM/HCTZ 20/12.5). The patients were randomized to receive either OM/AML/HCTZ 20/5/12.5 or OM/HCTZ 20/12.5 once daily for 8 weeks. At the end of 8 weeks, patients with uncontrolled BP were assigned to receive either OM/AML/HCTZ 40/5/12.5 or OM/AML/HCTZ 20/5/12.5 in an additional 8-week open-label extension period.
Results
A total of 623 patients received a 4-week run-in treatment with OM/HCTZ, 341 patients were randomized, and finally, 167 patients in the OM/AML/HCTZ group and 171 patients in the OM/HCTZ group were analyzed for the full analysis set. Non-responders after the 8 weeks of double-blind treatment continued the 8-week open-label treatment with OM/AML/HCTZ 40/5/12.5 mg (n = 32) or OM/AML/HCTZ 20/5/12.5 mg (n = 71). After 8 weeks of double-blind treatment, the changes in msDBP were −9.50 (8.46) mmHg in the OM/AML/HCTZ group and −4.23 (7.41) mmHg in the OM/HCTZ group (both p < 0.0001 vs. baseline; p < 0.0001 between groups). The response rates for both msSBP and msDBP at week 8 were 65.27 % in the OM/AML/HCTZ group and 37.43 % in the OM/HCTZ group (p < 0.0001 between groups). The response rates for both msSBP and msDBP at week 16 after open-label treatment were 18.75 % in the OM/AML/HCTZ 40/5/12.5 group and 46.48 % in the OM/AML/HCTZ 20/5/12.5 group (p = 0.0073 between groups). All medications were well tolerated.
Conclusion
In Korean patients with moderate hypertension not controlled with dual FDC therapy (OM/HCTZ 20/12.5) as first-line therapy, switching to triple FDC therapy (OM/AML/HCTZ 20/5/12.5) was associated with significant BP reductions and greater achievement of BP goals, and was well tolerated (ClinicalTrials.gov Identifier: NCT01838850).
Similar content being viewed by others
In Korean patients with moderate hypertension not controlled with dual fixed-dose combination (FDC) as first-line therapy, switching to triple FDC therapy is safe and effective in reaching target blood pressure. |
Triple FDC therapy can be a safe and effective alternative for Asian patients with hypertension not controlled with a dual FDC, including thiazide, in real-world clinical practice. |
1 Introduction
Most patients may need more than one antihypertensive drug to achieve target blood pressure (BP). Initial treatment with dual antihypertensive therapy is recommended for some patients, such as those with a markedly elevated BP or high/very high cardiovascular risk [1, 2]. In addition, almost half of patients prescribed an antihypertensive medication discontinued the treatment by the end of 1 year [3]. A single-pill combination therapy could simplify the antihypertensive regimen, particularly in patients with various comorbidities, which may improve compliance, persistence, and BP control compared with its corresponding free-drug combinations [2, 4, 5].
Guidelines for the management of hypertension from the American Society of Hypertension, the International Society of Hypertension, the Korean Society of Hypertension, the Japanese Society of Hypertension, and other meta-analysis data favor the use of combinations of two antihypertensive drugs at fixed doses in a single tablet [fixed-dose combination (FDC)] because reducing the number of pills improves adherence and increases the rate of BP control, minimizing adverse effects [1, 4, 6–9]. Different FDCs of the same two or three drugs are increasingly becoming available.
Antihypertensive agents with a combination of the angiotensin receptor blocker (ARB) olmesartan medoxomil (OM), the calcium antagonist amlodipine (AML), and the diuretic hydrochlorothiazide (HCTZ) are now available as an FDC tablet (OM/AML/HCTZ). The triple combination regimen with OM/AML/HCTZ, including high-dose OM 40 mg, was associated with significant BP reductions compared with any of the dual combination regimens at week 12 in the phase III TRINITY (triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension) study [10, 11]. According to the subgroup analyses of the TRINITY trial, OM/AML/HCTZ was more effective for BP reductions than each of the dual regimens, irrespective of hypertension severity, age, or sex [10, 12]. Other clinical studies reported that a triple combination of OM/AML/HCTZ provides effective BP reduction in patients whose BP is not controlled with a dual combination of OM/AML [13, 14]. However, data are limited regarding the efficacy and safety of dual FDC therapy in comparison with triple FDC therapy for reducing BP in their standard or low doses, rather than the higher or maximum doses used in the clinical trials, as a first-line therapy in actual clinical practice. The issue that triple FDC therapy can be a safe alternative for patients with hypertension not controlled with a dual FDC, including thiazide, is still unresolved.
The purpose of this study was to evaluate the efficacy and safety of low-dose triple FDC therapy with OM 20 mg, AML 5 mg, and HCTZ 12.5 mg (OM/AML/HCTZ 20/5/12.5) in Korean patients with moderate hypertension not controlled with low-dose dual FDC therapy with OM 20 mg and HCTZ 12.5 mg (OM/HCTZ 20/12.5) (ClinicalTrials.gov Identifier: NCT01838850).
2 Patients and Methods
2.1 Study Population
Korean men and women aged 20–75 years who had hypertension were screened for eligibility between April 2013 and January 2014. They were included in the study if they had newly diagnosed hypertension or had not undergone treatment with antihypertensive drugs within 4 weeks of screening, with a mean seated diastolic BP (msDBP) ≥100 mmHg at screening. Patients who had been receiving a stable dose of antihypertensive drugs for at least 4 weeks before the run-in period and met the following BP criteria at screening were also included: monotherapy, msDBP ≥95 mmHg; dual combination therapy, msDBP ≥90 mmHg; triple combination therapy, 70 mmHg ≤ msDBP < 90 mmHg. Patients whose mean seated systolic BP (msSBP) was ≥140 mmHg (msSBP ≥130 mmHg in subjects with diabetes or chronic kidney disease) and whose msDBP was ≥90 mmHg (msDBP ≥80 mmHg in subjects with diabetes or chronic kidney disease) were randomized.
Patients were excluded if they had an msDBP ≥115 mmHg or msSBP ≥200 mmHg measured at screening and randomization, a minimum–maximum difference in seated SBP of ≥20 mmHg or seated DBP of ≥10 mmHg in the chosen arm at screening, or a difference in seated SBP of ≥20 mmHg and seated DBP of ≥10 mmHg in both arms at screening. Patients were also excluded if they were hypersensitive to the investigational product or any of its components; had a medical history of hypersensitivity to sulfonamide, dihydropyridine, or thiazide diuretics; had a history of secondary hypertension or any of the diseases suspected of secondary hypertension, symptomatic orthostatic hypotension, uncontrolled diabetes mellitus (fasting blood sugar level >200 mg/dl), severe symptomatic heart failure, ischemic heart disease, or peripheral vascular disease; had undergone interventions within 6 months before screening; had clinically significant arrhythmias (ventricular tachycardia, atrial fibrillation, atrial flutter, or other arrhythmia considered clinically significant), hypertrophic obstructive cardiomyopathy, hemodynamically significant stenosis on the aortic valve or mitral valve, severe cerebrovascular disorder, known moderate or malignant retinopathy, any known autoimmune disease, or connective tissue disease; required chronic anti-inflammatory treatment; had anuria or severe renal failure, severe hepatic failure, biliary obstruction, biliary cirrhosis, or cholestasis, Addison’s disease, glucose–galactose malabsorption, galactose intolerance, or Lapp lactase deficiency; had gastrointestinal tract disease or undergone a surgical operation that may affect absorption, distribution, metabolism, and excretion of drugs; had active gastritis or gastrointestinal/rectal bleeding considered clinically significant by the investigator; had active inflammatory bowel syndrome within the last 12 months; had a history of or were suspected of drug or alcohol abuse; were pregnant or lactating; or were women of childbearing potential who did not agree to use appropriate contraceptive methods such as progestin hormone therapy (oral, implant), intrauterine device, barrier methods of contraception [condom or occlusive cap (diaphragm or cervical/vault caps) with spermicide], male sterilization, or true abstinence.
2.2 Study Design and Procedures
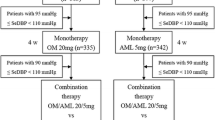
This 16-week, multicenter, randomized, double-blind, parallel-group study was conducted at 39 locations in the Republic of Korea. Participants who received OM/HCTZ 20/12.5 mg for the 4-week run-in period but who did not meet their BP goals (msSBP/msDBP <140/90 mmHg or msSBP/msDBP <130/80 mmHg in subjects with diabetes or chronic kidney disease; non-responders) could start receiving either the triple FDC therapy with OM/AML/HCTZ 20/5/12.5 mg or the dual FDC with OM/HCTZ 20/12.5 mg and a matched placebo during a randomized, 8-week, double-blind period. The non-responders after the 8 weeks of treatment could continue the 8-week open-label period with switching to OM/AML/HCTZ 40/5/12.5 mg from OM/AML/HCTZ 20/5/12.5 mg or OM/AML/HCTZ 20/5/12.5 mg from OM/HCTZ 20/12.5 mg (Fig. 1).
BP was measured at all study visits with a cuff of an appropriate size by using standard mercury sphygmomanometer calibrated on a regular basis at each study sites. Measurements were taken with the patient in a seated position after a 5-min rest. BP measurements were obtained three times at 2-min intervals in both arms. The arm was chosen if the BP was higher than the other arm. The mean of the last two measurements was recorded as the BP value for that visit. Patients were excluded from the study if msDBP was ≥115 mmHg or msSBP was ≥200 mmHg at screening and randomization. All of the patients provided informed consent, and the study protocol was approved by the institutional review board at each location. This study was prospectively registered at ClinicalTrials.gov: Identifier NCT01838850.
2.3 Efficacy Assessment
The primary endpoint was the change in msDBP from randomization to the end of the 8-week double-blind treatment period for triple FDC therapy compared with dual FDC therapy. The secondary endpoints included the change in the msSBP from baseline to the end of the 8-week treatment; changes in the msDBP and msSBP from randomization to 4 weeks of treatment; the percentage of patients who achieved their BP goal (msSBP/msDBP <140/90 mmHg or msSBP/msDBP <130/80 mmHg with diabetes or chronic kidney disease) at weeks 4, 8, and 16; and the change in msDBP and msSBP from week 8 to week 16 during the open-label treatment period. Responders were defined as patients who reached the BP goal at week 8, and non-responders were those who did not.
2.4 Safety Assessment
Safety assessments included adverse events (AEs), clinical laboratory examinations (chemistry, hematology, and urinalysis), vital signs (seated BP and seated heart rate), physical examinations, and 12-lead electrocardiographs. Laboratory tests were performed at each study site and were assessed before screening, at day 0, and at weeks 4, 8, and 16. Seated BP and heart rate were measured, and physical examinations were performed at all study visits. Twelve-lead electrocardiographs were obtained at the screening visit and at weeks 8 and 16. Patient adherence was monitored by assessing the tablet count from drug packages returned at each visit. The severity of AEs (mild, moderate, or severe) and their relationship to treatment (certainly related, probably related, possibly related, unlikely to be related, or not related to the study drug) were assessed and reported based on the judgment of the investigators.
2.5 Statistical Analysis
The hypothesis in this study was that triple FDC therapy with OM/AML/HCTZ is superior to dual FDC with OM/HCTZ in reducing msDBP. The mean change in DBP in the TRINITY study was −16.5 ± 10.8 mmHg with dual FDC therapy (OM/HCTZ) and −21.5 ± 10.3 mmHg with triple FDC therapy (OM/AML/HCTZ), resulting in an effect size of −5 mmHg for superiority margin [10]. In general, the smallest effect size was −2 mmHg between the treatment groups in most clinical trials regarding FDC therapy [15]. We selected −3.5 mmHg as the median value between −5 and −2 mmHg and as the superiority margin for the difference in the msDBP between treatments. The pooled standard deviation (SD) from the TRINITY study was considered to be 10.55 mmHg. All parameters were assumed as follows: the superiority margin for the difference in the msDBP between treatments (ε) = −3.5 mmHg; significance level (α) = 0.05; power of 0.80 (β = 0.2); and standard deviation (σ) = 10.55 mmHg. An estimated sample size of 143 patients per treatment group would be required, and 338 patients (169 per treatment group) were needed, considering a dropout rate of 15 %.
For continuous variables, the mean (SD), median, and minimum and maximum values were determined and compared using the independent t test or Wilcoxon rank-sum test between groups and the paired t test or Wilcoxon signed-rank test within a group. For categorical demographic variables, absolute and relative frequencies were determined and compared using the χ 2 test or Fisher’s exact test. The absolute and relative frequencies of msSBP <140 mmHg, msDBP <90 mmHg, or both, and the response rates at weeks 4, 8, and 16 of treatment were determined and compared using the χ 2 test or Fisher’s exact test. The least-squares mean changes in the msDBP were analyzed with analysis of covariance. Statistical significance was defined as a p < 0.05. No adjustments were made for multiplicity. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Study Population
A total of 662 patients were screened; 623 patients underwent a 4-week run-in treatment with OM/HCTZ, 209 patients achieved their BP goals (209/623, 33.6 %), 341 patients were randomized to either the OM/AML/HCTZ group (n = 169) or the OM/HCTZ group (n = 172), and 316 patients completed the double-blind treatment. Two patients in the OM/AML/HCTZ group and one patient in the OM/HCTZ group were not assessed at the primary endpoint. Therefore, 167 patients in the OM/AML/HCTZ group and 171 patients in the OM/HCTZ group were analyzed for the full analysis set. The non-responders after the 8 weeks of treatment (32 patients in the OM/AML/HCTZ group and 73 patients in the OM/HCTZ group) continued the 8-week open-label treatment with OM/AML/HCTZ 40/5/12.5 mg or OM/AML/HCTZ 20/5/12.5 mg, respectively (Fig. 1). The baseline demographic and clinical characteristics, including age, sex, weight, height, body mass index, smoking status, alcohol intake, duration of hypertension, presence of family history of hypertension, prevalence of diabetes and chronic kidney disease, and antihypertensive drug history did not differ significantly between the two groups (Table 1). Medication adherence appeared to be similar across treatment groups, ranging from 96.3 to 98.5 %.
3.2 Efficacy
After 8 weeks of double-blind treatment, the changes in msDBP were −9.50 (8.46) mmHg in the OM/AML/HCTZ group and −4.23 (7.41) mmHg in the OM/HCTZ group (both p < 0.0001 vs. baseline; p < 0.0001 between groups; Fig. 2a). The changes in msSBP from baseline to the end of the 8-week treatment were −16.30 (12.37) mmHg in the OM/AML/HCTZ group and −9.01 (12.90) mmHg in the OM/HCTZ group (both p < 0.0001 vs. baseline; p < 0.0001 between groups; Fig. 2b).
The changes in msDBP and msSBP from randomization to after 4 weeks of treatment were −11.39 (8.34) mmHg and −14.75 (12.50) mmHg, respectively, in the OM/AML/HCTZ group (both p < 0.0001 vs. baseline) and −5.74 (9.22) mmHg and −7.93 (11.99) mmHg, respectively, in the OM/HCTZ group (both p < 0.0001 vs. baseline; both p < 0.0001 between groups; Fig. 2).
The percentages of the patients who achieved their BP goal for both msSBP and msDBP at week 4 were 53.89 % in the OM/AML/HCTZ group and 28.65 % in the OM/HCTZ group (p < 0.0001 between groups). The response rates for both msSBP and msDBP at week 8 were 65.27 % in the OM/AML/HCTZ group and 37.43 % in the OM/HCTZ group (p < 0.0001 between groups; Table 2).
The changes in msDBP from week 8 to week 16 during the open-label treatment period were −5.38 (8.86) mmHg in the OM/AML/HCTZ 40/5/12/5 group (p < 0.0017 vs. baseline) and −11.07 (8.22) mmHg in the OM/AML/HCTZ 20/5/12.5 group (p < 0.0001 vs. baseline; p < 0.0020 between groups). The changes in msSBP after 8 weeks of open-label treatment were −9.22 (10.33) mmHg in the OM/AML/HCTZ 40/5/12/5 group (p < 0.0001 vs. baseline) and −16.58 (13.66) mmHg in the OM/AML/HCTZ 20/5/12.5 group (p < 0.0001 vs. baseline; p < 0.0078 between groups; Fig. 3). The response rates for both msSBP and msDBP at week 16 were 18.75 % in the OM/AML/HCTZ 40/5/12.5 group and 46.48 % in the OM/AML/HCTZ 20/5/12.5 group (p = 0.0073 between groups; Table 2).
Changes in mean seated diastolic blood pressure (msDBP) (a) and mean seated systolic blood pressure (msSBP) (b) from week 8 to week 16 during the open-label period. AML amlodipine, HCTZ hydrochlorothiazide, OM olmesartan medoxomil. *p = 0.0434, † p = 0.1650, ‡ p = 0.2012, § p = 0.1085 vs. OM/AML/HCTZ 20/5/12.5, respectively
The least-squares mean changes in msDBP from randomization to after 8 weeks of double-blind treatment were not significantly different according to sex, age, smoking status, and body mass index (Fig. 4).
3.3 Safety
Excluding one patient in the OM/AML/HCTZ group (due to the lack of detailed safety information), we assessed the safety profile in 340 patients (168 in the OM/AML/HCTZ group and 172 in the OM/HCTZ group). The overall incidence of AEs was relatively low and not significantly different in both treatment groups, and most AEs were mild (81 of 340 patients, 23.82 %) and not considered to be drug related (83 of 340 patients, 24.41 %; Table 3). A total of 46 AEs in the OM/AML/HCTZ group and 51 in the OM/HCTZ group were recorded. Five patients in the OM/AML/HCTZ group (2.98 %) and six patients in the OM/HCTZ group (3.49 %) experienced six and eight adverse drug reactions, respectively. Three patients experienced serious AEs. In the OM/AML/HCTZ group, one patient had acute tonsillitis and another underwent minor surgery for a cervical polyp. A patient in the OM/HCTZ group died after sudden cardiac arrest with hyperkalemia and with the probable diagnosis of acute myocardial infarction (described and reported as not related to the study drug by the investigator).
Discontinuations due to AEs occurred in four (2.38 %) of 168 patients in the OM/AML/HCTZ group. The reasons for discontinuation, each of which occurred in one patient (0.6 %), were dizziness, fatigue, and palpitation. Three other patients reported hypotension, pre-syncope, or cough, respectively, as a cause of discontinuation. Four (2.33 %) of 172 patients in the OM/HCTZ group discontinued their medications because of constipation, dizziness, or headache for (n = 3 patients each), and one patient died, as described previously.
4 Discussion
In the present study, we found that Korean patients with moderate hypertension who did not achieve their BP goals after the 4-week treatment with initial low-dose dual FDC therapy (OM/HCTZ 20/12.5 mg) showed significant improvement in BP control after switching to low-dose triple FDC therapy (OM/AML/HCTZ 20/5/12.5 mg), which was well tolerated. Among the non-responders of the 4-week run-in treatment who were randomized and switched to triple FDC therapy during the 8-week double-blind treatment, the percentage of patients who achieved their BP goal at week 8 was about two-thirds (65.27 %), but only about one-third (37.43 %) of the patients who continued dual FDC therapy achieved their target BP. For tolerability, only 8 of 340 patients (2.35 %) discontinued their medications.
Initiating antihypertensive therapy with a combination of two drugs is associated with a reduced risk of medication discontinuation [16]. The other advantages of this approach are the maximized BP reduction in the patients with markedly elevated BP, rapid BP control (especially in patients at high cardiovascular risk), and minimized adverse effects [2, 4]. Most guidelines favor the combination of an angiotensin-converting enzyme inhibitor or ARB, and thiazide diuretic or calcium antagonist [2, 4, 9]. They also recommend the use of FDCs because reducing the pill burden improves adherence and increases the BP control rate. In addition, to overcome the inconvenience and difficulty in increasing the dose of one drug in FDC therapy, different FDCs with different doses of each component are available.
When initiating a combination of two drugs, doses can be increased to achieve the BP target, or adding a third drug from different classes can be considered. A meta-analysis showed that combining two agents from any classes of antihypertensive drugs increases the BP reduction much more than increasing the dose of one drug, and the reduction in BP may be approximately fivefold greater with two agents than with doubling the dose of one agent [6]. In this study, we tried to test the efficacy and safety of ‘standard or low-dose’ triple FDC (OM/AML/HCTZ 20/5/12.5) in patients with moderate hypertension not controlled with ‘initial low-dose’ dual FDC therapy (OM/HCTZ 20/12.5), not with ‘maximum or high doses’ as done in the TRINITY trial [10]. In addition, the entire TRINITY trial population only included about 2 % Asians, and their mean body mass index was 33 kg/m2 [10]. In contrast, our study enrolled only Koreans (all Asians) who were much smaller (mean body mass index, 26.7 kg/m2) than the westerners, which means that in real practice Asians may need lower doses of anti-hypertensive drugs than larger-sized westerners. Because FDC therapy is less expensive than the total price of each single drug and because FDC therapy as a first-line therapy is covered by health insurance, FDC therapy has a combined medical and economic advantage for patients with hypertension in South Korea.
We chose OM with HCTZ as first-line FDC therapy because FDC therapy including an angiotensin-converting enzyme inhibitor is not available in South Korea. Second, an ARB combined with a diuretic rather than a calcium antagonist showed more clinical benefits than combined placebo or beta-blocker and diuretic in randomized controlled trials [17–19]. In addition, the combination of an ARB with a diuretic has advantages because of the synergistic BP-lowering effects and because an ARB offsets the adverse effects of a diuretic on electrolyte and glucose metabolism. In this study, among 623 patients who underwent the 4-week run-in treatment with dual FDC therapy (OM/HCTZ 20/12.5), 209 achieved their BP goals (209/623, 33.6 %). Compared with dual FDC therapy, triple FDC therapy was associated with a significantly higher percentage of patients who achieved their BP goal when the BP was not controlled with dual FDC therapy as first-line therapy. Interestingly, among the non-responders after the 8-week double-blind treatment who were allocated to triple FDC therapy (OM/AML/HCTZ) with a double dose of ARB OM, the response rate was less than one-fifth (18.75 %). In contrast, among the patients who received triple FDC therapy, including the calcium antagonist (AML) switched from double FDC therapy (OM/HCTZ), about one-half (46.48 %) achieved their target BP. This may suggest that patients who are not responsive to dual FDC therapy, including an ARB and a thiazide, could be switched to triple FDC therapy by adding a different class of drug (a calcium antagonist) for a safe and more effective BP reduction than doubling the dose of an agent.
4.1 Study Limitations
Our study was limited by its short treatment period and relatively small cohort size. Therefore, the safety results should be interpreted with caution, and a large-scale study may be needed to determine the long-term safety and clinical outcomes of triple FDC therapy.
5 Conclusions
In Korean patients with moderate hypertension not controlled with dual FDC therapy with OM/HCTZ 20/12.5 as a first-line therapy, switching to triple FDC therapy with OM/AML/HCTZ 20/5/12.5 was associated with significant BP reductions and greater achievement of BP goals, and was well tolerated. These findings may provide useful information to clinicians in choosing triple FDC therapy in Asian patients with moderate hypertension not controlled with dual FDC therapy for safe and effective BP reduction in actual clinical practice.
References
Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32(1):3–15. doi:10.1097/HJH.0000000000000065.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357. doi:10.1097/01.hjh.0000431740.32696.cc.
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–7. doi:10.1136/bmj.39553.670231.25.
Shin J, Park JB, Kim KI, Kim JH, Yang DH, Pyun WB, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension. Part II-treatments of hypertension. Clin Hypertens. 2015;21(2):1–13. doi:10.1186/s40885-014-0014-1.
Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi:10.1136/bmj.38875.675486.55.
Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290–300. doi:10.1016/j.amjmed.2008.09.038.
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55(2):399–407. doi:10.1161/HYPERTENSIONAHA.109.139816.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–310.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253–390. doi:10.1038/hr.2014.20.
Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: The TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32(7):1252–69. doi:10.1016/j.clinthera.2010.07.008.
Chrysant SG, Littlejohn T 3rd, Izzo JL Jr, Kereiakes DJ, Oparil S, Melino M, et al. Triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide in black and non-black study participants with hypertension: the TRINITY randomized, double-blind, 12-week, parallel-group study. Am J Cardiovasc Drugs. 2012;12(4):233–43. doi:10.2165/11634160-000000000-00000.
Lewin AJ, Izzo JL Jr, Melino M, Lee J, Fernandez V, Heyrman R. Combined olmesartan, amlodipine, and hydrochlorothiazide therapy in randomized patients with hypertension: a subgroup analysis of the TRINITY study by age. Drugs Aging. 2013;30(7):549–60. doi:10.1007/s40266-013-0072-1.
Chrysant SG, Oparil S, Melino M, Karki S, Lee J, Heyrman R. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens. 2009;11(9):475–82. doi:10.1111/j.1751-7176.2009.00159.x.
Volpe M, Miele C, Haag U. Efficacy and safety of a stepped-care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate-to-severe hypertension: an open-label, long-term study. Clin Drug Invest. 2009;29(6):381–91. doi:10.2165/00044011-200929060-00002.
Lange S, Freitag G. Choice of delta: requirements and reality—results of a systematic review. Biom J. 2005;47(1):12–27 (discussion 99–107).
Corrao G, Parodi A, Zambon A, Heiman F, Filippi A, Cricelli C, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step. Evidence from daily life practice. J Hypertens. 2010;28(7):1584–90. doi:10.1097/HJH.0b013e328339f9fa.
Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–86. doi:10.1097/01.hjh.0000059028.82022.89.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi:10.1016/S0140-6736(02)08089-3.
Kereiakes DJ, Neutel JM, Punzi HA, Xu J, Lipka LJ, Dubiel R. Efficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylate. Am J Cardiovasc Drugs. 2007;7(5):361–72.
Acknowledgments
All authors were responsible for the study design, data collection, and data interpretation. Drs. Sohn and Kim were responsible for data interpretation and writing the manuscript. We are grateful to Ms. Bo Young Cho for her dedicated assistance in statistics.
Study investigators: Chang-Wook Nam, MD, Keimyung University Dongsan Medical Center; Cheol-Ho Kim, MD, Dong-Ju Choi, MD, Seoul National University Bundang Hospital; Sang-Hong Baek, MD, Seoul St. Mary’s Hospital of the Catholic University of Korea; Woo-Baek Chung, MD, Yeouido St. Mary’s Hospital of the Catholic University of Korea; Woo-Shik Kim, MD, Kyung Hee University Medical Center; Tae-Hoon Ahn, MD, Gachon University Gil Medical Center; Jang-Hyun Cho, MD, St. Carollo Hospital; Byung-Hee Oh (Chair), MD, Seoul National University Hospital; Hweung-Kon Hwang, MD, Konkuk University Medical Center; Chang-Gyu Park, MD, Korea University Guro Hospital; Eun-Seok Shin, MD, Ulsan University Hospital; Joon-Han Shin, MD, Ajou University Hospital; Myung-Ho Jeong, MD, Chonnam National University Hospital; Jin-Ok Jeong, MD, Chungnam National University Hospital; Chong-Jin Kim, MD, Il Suk Sohn, MD, Kyung Hee University Hospital at Gangdong; Jang-Ho Bae, MD, Konyang University Hospital; Seung-Hwan Lee, MD, Wonju Severance Christian Hospital; Se-Joong Rim, MD, Gangnam Severance Hospital; Jay-Young Rhew, MD, Presbyterian Medical Center; Doo-Il Kim, MD, Inje University Haeundae Paik Hospital; Dae-Kyeong Kim, MD, Inje University Busan Paik Hospital; Soon-Kil Kim, MD, Hanyang University Guri Hospital; Hye-Sun Seo, MD, Soonchunhyang University Hospital; Duk-Hyun Kang, MD, Asan Medical Center; Young-Dae Kim, MD, Dong-A University Hospital; Dong-Woon Kim, MD, Chungbuk National University Hospital; Taek-Jong Hong, MD, Pusan National University Hospital; Jong-Won Ha, MD, Severance Hospital; Woo-Jung Park, MD, Hallym University Medical Center; Tae Ho Kim, MD, Chung-Ang University Hospital; Kee-Sik Kim, MD, Daegu Catholic University Medical Center; Seung-Woo Park, MD, Samsung Medical Center; Wan-Joo Shim, MD, Korea University Anam Hospital; Joo-Young Yang, MD, Health Insurance Service Ilsan Hospital; Jae-Woong Choi, MD, Eulji General Hospital; Sun-Hwa Lee, MD, Chonbuk National University Hospital; Jeong-Cheon Ahn, MD, Korea University Ansan Hospital; Keun Lee, MD, Seoul Veterans Hospital; Byung-Soo Kim, MD, Daedong Hospital.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
This study was sponsored by Daiichi-Sankyo Korea Co. Ltd, Seoul, Republic of Korea. Daiichi-Sankyo Korea was not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
Additional information
The members of the Investigators are listed in Acknowledgments.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sohn, I.S., Kim, CJ., Oh, BH. et al. Efficacy and Safety Study of Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide Combination Therapy in Patients with Hypertension Not Controlled with Olmesartan Medoxomil and Hydrochlorothiazide Combination Therapy: Results of a Randomized, Double-Blind, Multicenter Trial. Am J Cardiovasc Drugs 16, 129–138 (2016). https://doi.org/10.1007/s40256-015-0156-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-015-0156-x