Abstract
Background and objectives
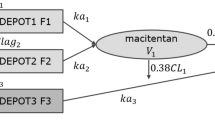
Macitentan is a novel dual endothelin (ET)-1 receptor antagonist to be used in patients with pulmonary arterial hypertension. This study aimed to assess the pharmacokinetics (PK) and pharmacodynamics (PD) of macitentan after administration of multiple doses to healthy Korean male subjects.
Methods
A randomized, double-blind, placebo-controlled, multiple-ascending dose study was performed in 30 healthy male subjects receiving oral macitentan (3, 10, or 30 mg) or placebo once daily for 10 days. Plasma concentrations of macitentan, its active metabolite ACT-13277, and ET-1 were evaluated. Safety and tolerability measurements were conducted throughout the study.
Results
The concentration–time profile of macitentan was characterized by slow absorption (median time to maximum plasma concentration [t max] 9–10 h) and slow elimination (mean elimination half-life [t ½] 11–15 h). After repeated doses of 3, 10, and 30 mg of macitentan over the course of 10 days, the peak concentration (C max) increased as the dose increased and the area under the plasma concentration–time curve during the dosing interval (AUC τ ) increased in a dose-proportional manner. Plasma concentrations showed approximately 1.5- to 1.9-fold accumulation on day 10 compared with day 1. ACT-132577 showed higher levels of exposure than macitentan, its mean half-life was 46–48 h, and it accumulated 7- to 12-fold. Macitentan increased plasma ET-1 concentrations at all doses tested and was well tolerated and elicited no serious adverse events.
Conclusion
Multiple oral doses of 3, 10, and 30 mg of macitentan were well tolerated in healthy Korean subjects, and its pharmacokinetics correlated positively with ET-1 concentrations.



Similar content being viewed by others
Reference
Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev. 2012;21(126):321–7.
Webb DJ. Endothelin: from molecule to man. Br J Clin Pharmacol. 1997;44(1):9–20.
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5.
Anderson JR, Nawarskas JJ. Pharmacotherapeutic management of pulmonary arterial hypertension. Cardiol Rev. 2010;18(3):148–62.
Price LC, Howard LS. Endothelin receptor antagonists for pulmonary arterial hypertension: rationale and place in therapy. Am J Cardiovasc Drugs. 2008;8(3):171–85.
Benigni A, Remuzzi G. Endothelin antagonists. Lancet. 1999;353(9147):133–8.
Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52(3):452–9.
Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69(4):223–31.
Don GW, Joseph F, Celermajer DS, Corte TJ. Ironic case of hepatic dysfunction following the global withdrawal of sitaxentan. Intern Med J. 2012;42(12):1351–4.
Galie N, Hoeper MM, Gibbs JS, Simonneau G. Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J. 2011;37(2):475–6.
Trow TK, Taichman DB. Endothelin receptor blockade in the management of pulmonary arterial hypertension: selective and dual antagonism. Respir Med. 2009;103(7):951–62.
Bolli MH, Boss C, Binkert C, Buchmann S, Bur D, Hess P, et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-propylsulfamide (macitentan), an orally active, potent dual endothelin receptor antagonist. J Med Chem. 2012;55(17):7849–61.
Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327(3):736–45.
Sidharta PN, van Giersbergen PL, Halabi A, Dingemanse J. Macitentan: entry-into-humans study with a new endothelin receptor antagonist. Eur J Clin Pharmacol. 2011;67(10):977–84.
Sidharta PN, van Giersbergen PL, Dingemanse J. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macitentan, an endothelin receptor antagonist, in an ascending multiple-dose study in healthy subjects. J Clin Pharmacol. 2013;53(11):1131–8.
Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–18.
Bruderer S, Marjason J, Sidharta PN, Dingemanse J. Pharmacokinetics of macitentan in Caucasian and Japanese subjects: the influence of ethnicity and sex. Pharmacology. 2013;91(5–6):331–8.
Dingemanse J, Sidharta PN, Maddrey WC, Rubin LJ, Mickail H. Efficacy, safety and clinical pharmacology of macitentan in comparison to other endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Expert Opin Drug Saf. 2014;13(3):391-405.
World Medical Association Declaration of Helsinki—ethical principles for medical research involving human subjects. http://www.wma.net/en/30publications/10policies/b3/indexhtml. Accessed 03 July 2013.
European Medicines Agency. ICH Topic E 6 (R1): guideline for good clinical practice. Note for guidance on good clinical practice. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002874.pdf. Accessed 03 July 2013.
Kummer O, Haschke M, Hammann F, Bodmer M, Bruderer S, Regnault Y, et al. Comparison of the dissolution and pharmacokinetic profiles of two galenical formulations of the endothelin receptor antagonist macitentan. Eur J Pharm Sci. 2009;38(4):384–8.
Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–83.
Bruderer S, Hopfgartner G, Seiberling M, Wank J, Sidharta PN, Treiber A, et al. Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans. Xenobiotica. 2012;42(9):901–10.
Bruderer S, Aanismaa P, Homery MC, Hausler S, Landskroner K, Sidharta PN, et al. Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist. AAPS J. 2012;14(1):68–78.
Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141(1):20–6.
Loffler BM, Breu V, Clozel M. Effect of different endothelin receptor antagonists and of the novel non-peptide antagonist Ro 46-2005 on endothelin levels in rat plasma. FEBS Lett. 1993;333(1–2):108–10.
Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346(8977):732–6.
Weber C, Schmitt R, Birnboeck H, Hopfgartner G, van Marle SP, Peeters PA, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60(2):124–37.
Perez-Urizar J, Granados-Soto V, Flores-Murrieta FJ, Castaneda-Hernandez G. Pharmacokinetic–pharmacodynamic modeling: why? Arch Med Res. 2000;31(6):539–45.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. FDA guidance for industry exposure–response relationships: study design, data analysis, and regulatory applications [online]. 2003 April. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf.
Dingemanse J, Gunawardena KA, van Giersbergen PL. Comparison of the pharmacokinetics, pharmacodynamics and tolerability of tezosentan between Caucasian and Japanese subjects. Br J Clin Pharmacol. 2006;61(4):405–13.
Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42(6):1622-32.
Acknowledgments
Sponsored by Actelion Pharmaceuticals Ltd, Korea, this study was designed and performed by qualified investigators of the Department of Clinical Pharmacology and Therapeutics of SNUH. Li Young Ahn, Sung Eun Kim, and SoJeong Yi received training program grants from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Korea (A070001, Korea National Enterprise for Clinical Trials).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahn, L.Y., Kim, S.E., Yi, S. et al. Pharmacokinetic–Pharmacodynamic Relationships of Macitentan, a New Endothelin Receptor Antagonist, After Multiple Dosing in Healthy Korean Subjects. Am J Cardiovasc Drugs 14, 377–385 (2014). https://doi.org/10.1007/s40256-014-0081-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-014-0081-4