Abstract
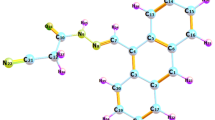
A density function theory(DFT) study was made on three dyes based on hydroxamate with different ligands[terpyridine, isothiocyanate(NCS) and 2,2′-bis(thienyl)-tripyrrinate(2-BTTP)] to investigtate their device performance optimization in dye sensitized solar cell(DSSC). Based on the adsorbed dye on TiO2 (101) surface, the ground state geometry structures, electronic structures, absorption spectra and correspongding charge transfer properties were analysed in detail. The results indicate that the ligand replacement of terpyridine by NCS and 2-BTTP improves the low-energy region absorption of hydroxamate based dyes significantly. The electron injection and light harvesting capability of hydroxamate based dyes are enhanced by NCS and 2-BTTP ligands as well. In the visible region, hydroxamate based dyes have the potentials to become panchromatic light absorbers according to our research.
Similar content being viewed by others
References
O’regan B., Gräzel M., Nature, 1991, 353, 24
Hagfeldt A., Grätzel M., Acc. Chem. Res., 2000, 33, 269
Kalyanasundaram K., Grätzel M., Coord. Chem. Rev., 1998, 177, 347
Grätzel M., Nature, 2001, 414, 338
Nazeeruddin M. K., Pechy P., Renouard T., Zakeeruddin S. M., Humphry B. R., Comte P., Liska P., Cevey L., Costa E., Shklover V., J. Am. Chem. Soc., 2001, 123, 1613
Chen J., Wang J., Bai F. Q., Zheng Q. C., Zhang H. X., Chem. Res. Chinese Universities, 2012, 28(4), 696
Chen J., Wang M., Chem. Res. Chinese Universities, 2013, 29(3), 584
Zhao Y. D., Fu J. J., Li H. B., Dong H., Liao Y., Chem. Res. Chinese Universities, 2013, 29(5), 974
Nazeeruddin M. K., Kay A., Rodicio I., Humphry-Baker R., Müller E., Liska P., Vlachopoulos N., Grätzel M., J. Am. Chem. Soc., 1993, 115, 6382
Nazeeruddin M. K., Zakeeruddin S., Humphry B. R., Jirousek M., Liska P., Vlachopoulos N., Shklover V., Fischer C. H., Grätzel M., Inorg. Chem., 1999, 38, 6298
Onozawa-Komatsuzaki N., Yanagida M., Funaki T., Kasuga K., Sayama K., Sugihara H., Sol. Energ. Mat. Sol. C, 2011, 95, 310
Kusama H., Sayama K., J. Phys. Chem. C, 2012, 116, 1493
Chen C. Y., Wu S. J., Wu C. G., Chen J. G., Ho K. C., Angew. Chem., 2006, 118, 5954
Chen J., Bai F. Q., Wang J., Sun L., Pan Q. J., Zhang H. X., Science China Chemistry, 2012, 55, 398
Dai F. R., Wu W. J., Wang Q. W., Tian H., Wong W. Y., Dalton Trans., 2011, 40, 2314
Numata Y., Singh S. P., Islam A., Iwamura M., Imai A., Nozaki K., Han L., Adv. Funct. Mater., 2013, 23, 1817
Chou C. C., Wu K. L., Chi Y., Hu W. P., Yu S. J., Lee G. H., Lin C. L., Chou P. T., Ang. Chem. Int. Ed., 2011, 50, 2054
Wu K. L., Li C. H., Chi Y., Clifford J. N., Cabau L., Palomares E., Cheng Y. M., Pan H. A., Chou P. T., J. Am. Chem. Soc., 2012, 134, 7488
Brewster T. P., Konezny S. J., Sheehan S. W., Martini L. A., Schmuttenmaer C. A., Batista V. S., Crabtree R. H., Inorg. Chem., 2013, 52, 6752
Koenigsmann C., Ripolles T., Brennan B., Negre C., Koepf M., Durrell A., Milot R., Torre J., Crabtree R. H., Batista V., Phys. Chem. Chem. Phys., 2014, 16, 16629
Li G., Bomben P. G., Robson K. C., Gorelsky S. I., Berlinguette C. P., Shatruk M., Chem. Commun., 2012, 48, 8790
Li G., Ray L., Glass E. N., Kovnir K., Khoroshutin A., Gorelsky S. I., Shatruk M., Inorg. Chem., 2012, 51, 1614
Robertson N., Angew. Chem. Int. Ed., 2006, 45, 2338
Stergiopoulos T., Karakostas S., Falaras P., Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163, 331
Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G.E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09. D01, Gaussian Inc., Wallingford CT, 2009
Chen J., Bai F. Q., Wang J., Hao L., Xie Z. F., Pan Q. J., Zhang H. X., Dyes Pigm., 2012, 94, 459
Becke A. D., J. Chem. Phy., 1993, 98, 5648
Hariharan P. C., Pople J. A., Theor. Chim. Acta, 1973, 28, 213
Check C. E., Faust T. O., Bailey J. M., Wright B. J., Gilbert T. M., Sunderlin L. S., J. Phys. Chem. A, 2001, 105, 8111
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.21173096) and the China Postdoctoral Science Foundation(No.2013M541288).
Rights and permissions
About this article
Cite this article
Xie, M., Chen, J., Wang, J. et al. Optical properties of dye based on hydroxamate improved with designed tridentate ligands for dye sensitized solar cell: a theoretical study. Chem. Res. Chin. Univ. 31, 830–834 (2015). https://doi.org/10.1007/s40242-015-5073-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-015-5073-7