Abstract
Introduction
Intragastric balloons (IGBs) have increased in popularity within the continuum of care for obesity. The FDA has approved 3 different devices with similar mechanisms of action and approved treatment durations. IGBs can offer help with weight loss to individuals with BMI 30–40 kg/m2 who do not qualify for bariatric surgery or who do not wish to undergo a surgical procedure for weight loss.
Methods
This is a review of available current literature regarding the efficacy and outcomes of IGBs for treatment of obesity and morbid obesity including randomized controlled trials (RCTs) and meta-analyses published from 2005 to 2017.
Results
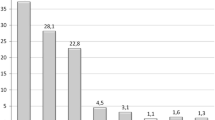
Five RCTs reported weight-loss results of IGB treatment in terms of percentage of excess weight loss (%EWL). Devices were placed for 3–12 months duration and %EWL ranged from 25.1 to 50.3% at device removal and 18.8–57% 6 months later. Three RCTs reported percentage of total weight-loss (%TWL) results with IGB durations from 6 to 12 months ranging from 10.2 to 17.1% at the time of device removal. Three meta-analyses reported %EWL of 27.4–36.2% within 6 months of IGB treatment.
Conclusion
IGBs have favorable safety profiles and have shown significant short-term weight-loss improvement over lifestyle modification or pharmacotherapy. Further investigation is required to determine the long-term benefit of these devices for patients with obesity and morbid obesity as well as to monitor the devices’ long-term safety profiles.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8.
Centers for Disease Control and Prevention: Adult Obesity Causes and Consequences. https://www.cdc.gov/obesity/adult/causes.html (2017). Accessed 27 Sept 2017.
Gastrointestinal surgery for severe obesity. Consens Statement. 1991 Mar 25-27;9(1):1-20 9.
Buchwald H, Consensus Conference Panel. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1(3):371–81.
US Food and Drug Administration. Obalon Balloon System–P160001. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm520741.htm (2016). Accessed 26 Sept 2017.
US Food and Drug Administration. ORBERA™ Intragastric Balloon System—P140008. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm457416.htm. (2015). Accessed 26 Sept 2017.
US Food and Drug Administration. ReShape Integrated Dual Balloon System—P140012. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm456293.htm (2015). Accessed 26 Sept 2017.
US Food and Drug Administration. Obalon® Balloon System Level Information Manual. Accessed 26 Sept 2017.
•• Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, Ballem N, Kligman M, Kothari S; ASMBS Clinical Issues Committee. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–06. doi:10.1016/j.soard.2015.02.003. This paper outlines strategies for standardized outcomes reporting for manuscripts written about bariatric and metabolic surgery in order to allow different works to be more comparable to one another.
Genco A, Cipriano M, Bacci V, Cuzzolaro M, Materia A, Raparelli L, Docimo C, Lorenzo M, Basso N. BioEnterics Intragastric Balloon (BIB): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30(1):129–33 PubMed PMID: 16189503.
Peker Y, Coskun H, Bozkurt S, Cin N, Atak T, Genc H. Comparison of results of laparoscopic gastric banding and consecutive intragastric balloon application at 18 months: a clinical prospective study. J Laparoendosc Adv Surg Tech A. 2011;21(6):471–5. doi:10.1089/lap.2010.0439.
Fuller NR, Pearson S, Lau NS, Wlodarczyk J, Halstead MB, Tee HP, Chettiar R, Kaffes AJ. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity. 2013;21(8):1561–70. doi:10.1002/oby.20414.
Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9(2):290–5. doi:10.1016/j.soard.2012.07.007.
• Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F, Sullivan S, Holcomb R, Lehmann J; REDUCE Pivotal Trial Investigators. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11(4):874–81. doi:10.1016/j.soard.2014.12.006. The REDUCE trial enrolled a larger number of patients into an RTC and helped identify two issues with IGB devices. Decreasing the fill volumes based on patient body size helped decrease early removal rates. Secondly, findings allowed for modification of the device to include a softer tip that helped significantly reduce gastric ulcer rate and size.
Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1 year balloon treatment followed by a 1 year balloon-free follow-up. Gastrointest Endosc. 2005;61(1):19–27.
Farina MG, Baratta R, Nigro A, Vinciguerra F, Puglisi C, Schembri R, Virgilio C, Vigneri R, Frittitta L. Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg. 2012;22(4):565–71. doi:10.1007/s11695-011-0514-y.
Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76(4):756–60. doi:10.1016/j.gie.2012.05.023.
Courcoulas A, Abu Dayyeh BK, Eaton L, Robinson J, Woodman G, Fusco M, Shayani V, Billy H, Pambianco D, Gostout C. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes. 2017;41(3):427–33. doi:10.1038/ijo.2016.229.
Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18(7):841–6. doi:10.1007/s11695-007-9331-8.
Zheng Y, Wang M, He S, Ji G. Short-term effects of intragastric balloon in association with conservative therapy on weight loss: a meta-analysis. J Transl Med. 2015;29(13):246. doi:10.1186/s12967-015-0607-9.
Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, Gill R, Karmali S. Intragastric balloon for management of severe obesity: a systematic review. Obes Surg. 2016;26(9):2248–54. doi:10.1007/s11695-016-2307-9.
• Maisel, W MD, MPH. The FDA alerts health care providers about potential risks with liquid-filled intragastric balloons. U.S. Food & Drug Administration. https://www.fda.gov/MedicalDevices/ResourcesforYou/HealthCareProviders/ucm540655.htm (2017). Accessed 26 Sept 2017. This letter from the FDA identified complications of IGBs including overinflation and pancreatitis that can both be significant health risks if untreated. Increasing awareness of IGB related complications to physicians caring for these patients including primary care and emergency departments should help to identify problems sooner and allow quicker access to necessary treatment, often including early device removal.
US Food and Drug Administration. UPDATE: Potential risks with liquid-filled intragastric balloons—Letter to Health Care Providers. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm570707.htm (2017). 26 Sept 2017.
Raftopoulos I, Giannakou A. The Elipse Balloon, a swallowable gastric balloon for weight loss not requiring sedation, anesthesia or endoscopy: a pilot study with 12 month outcomes. Surg Obes Relat Dis. 2017;13(7):1174–82. doi:10.1016/j.soard.2017.02.016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Bariatric Surgery.
Rights and permissions
About this article
Cite this article
Vosburg, R.W., Kim, J. Intragastric Balloons: Indications, Options, Outcomes. Curr Surg Rep 5, 34 (2017). https://doi.org/10.1007/s40137-017-0197-y
Published:
DOI: https://doi.org/10.1007/s40137-017-0197-y