Abstract
Introduction
Anti-staphylococcal penicillins are generally accepted as first-line therapy for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, but their use may be limited by interstitial nephritis and acute kidney injury. Alternatives include first-generation cephalosporins including cefazolin.
Methods
We conducted a retrospective cohort study to compare adverse effects and clinical outcomes among patients with MSSA bacteremia treated with cefazolin or nafcillin. The primary endpoint was acute kidney injury (AKI), defined as a 0.3 mg/dL or 50% increase from baseline.
Results
Incidence of AKI was 27/82 (33%) versus 9/68 (13%) (p = 0.007) in the nafcillin and cefazolin arms, respectively. After adjusting for endocarditis and intensive care unit admission in multivariate logistic regression, nafcillin was an independent predictor of AKI [adj odds ratio (OR) = 2.74; 95% (CI) 1.1–6.6]. Patients who experienced AKI were more likely to have a prolonged intensive care unit stay.
Conclusion
Risk of nephrotoxicity is increased with nafcillin compared with cefazolin. Cefazolin should considered as a safer alternative to nafcillin for select patients with MSSA bacteremia.
Similar content being viewed by others
Introduction
Staphylococcus aureus is a pathogen that is associated with high healthcare costs and mortality. When compared to other organisms causing bacteremia, it was associated with a greater median length of stay and total treatment cost [1]. Although the incidence of methicillin-resistant S. aureus (MRSA) infections are rising, methicillin-susceptible S. aureus (MSSA) bloodstream infections (BSI) are still prevalent [2]. For the treatment of MSSA infections, use of beta-lactams antibiotics compared to vancomycin therapy is associated with about a 35% lower rate of mortality [3, 4]. Therefore, beta-lactams are known to be the treatment of choice for this infection. However, what is not well established is which beta-lactam is preferred.
Comparative studies of MSSA bacteremia suggest no overall difference in clinical success, mortality, or length of stay between anti-staphylococcal penicillins and cefazolin [5–9]. However, recent literature suggests that cefazolin may offer safety advantages. Nafcillin has been associated with a higher rate of premature antibiotic discontinuation, 18–33.8% due to adverse effects, compared with 2–6.7% for cefazolin. It has also been associated with a higher incidence of nephrotoxicity of 11.4–15% and cefazolin with 0–3.4% [10, 11]. Generalizability of the previous literature may be limited by the study setting of outpatient versus inpatient, definitions utilized, and early beta-lactam discontinuation. Definitions of AKI throughout the literature vary in sensitivity; however, if improving safety through early identification is the endpoint of interest, a more conservative definition is preferred [12].
Based on the literature to date, it appears that cefazolin may be a safer alternative [10, 11]. In addition, the average wholesale price of nafcillin for 1 day of treatment is approximately US$162 compared with less than $8 per day for cefazolin. Using a mean duration of treatment for MSSA bacteremia of 20 days, from the literature, the total medication cost would be $3400 with nafcillin and $166 for cefazolin [11]. A comprehensive economic analysis incorporating drug costs, toxicity, and cost of care has yet to be published. The purpose of this study was to evaluate the outcomes of infection, safety and economic impact of using cefazolin versus nafcillin in patients with MSSA BSI. We hypothesized that cefazolin would be associated with less nephrotoxicity and would be a more cost-effective alternative than nafcillin, but would have similar clinical outcomes.
Methods
This retrospective cohort study was conducted at the Henry Ford Health System, a four-hospital health system in Detroit, Michigan, and surrounding areas. This study was approved by the Henry Ford Hospital institutional review board. This article does not contain any new studies with human or animal subjects performed by any of the authors. Patients were eligible for inclusion if they were ≥18 years old with at least one positive MSSA blood culture from November 2013 to October 2015 and received cefazolin or nafcillin for ≥72 h. If patients received both nafcillin and cefazolin, then they were included in the arm corresponding to initial beta-lactam received ≥72 h. Patients were excluded if they had evidence of pre-existing renal dysfunction indicated by a serum creatinine (SCr) >2 mg/dL, any form of renal replacement therapy, or if meningitis was the suspected or confirmed source.
The primary outcome was the incidence of nephrotoxicity defined as an increase in SCr from baseline ≥0.3 mg/dL within 48 h, or any 50% increase as assessed by blinded adjudication [12]. Clinical failure was defined as all-cause mortality at 30 days, a recurrence of MSSA infection within 60 days of discharge, and microbiologic failure. Microbiologic failure was defined as blood cultures growing MSSA for >7 days from the start of initial beta-lactam therapy. Clinical success was determined if all components of the clinical failure definition were not met. In-hospital mortality deemed to be related to the MSSA infection included patients with blood cultures still positive or febrile on the day of death. Data was collected from the electronic medical record using a standardized case report form, and included patient demographics, comorbid conditions, risk factors for AKI, infection, and treatment characteristics.
Resource utilization measures included total length of stay (LOS), LOS in the intensive care unit, average wholesale price (AWP), and overall estimated cost of hospital stay. The health system's average cost per day in an intensive care unit and general practice unit according to American Hospital Association data and AWP were used for the analytical model.
IBM SPSS v.21 (Chicago, IL, USA) was utilized for statistical analysis. Categorical variables were compared with Pearson Chi square tests and continuous variables with Mann–Whitney U. Assuming an incidence of AKI of 30% in the nafcillin group, approximately 75 patients were required in each group to detect a difference of 20% in AKI with 80% power with an alpha-level of 0.05 [13]. A multivariable logistic regression analysis was used to evaluate the effect of nafcillin on nephrotoxicity after adjustment for confounding variables. Variables were considered for the regression analysis if p values were <0.2 in the univariate analysis or considered clinically relevant. A decision analysis was used to assess the most cost-effective treatment using TreeAge software (Williamstown, MA, USA).
Results
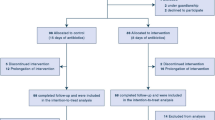
Of the 343 patients identified to have at least one positive culture for MSSA during the study period, a total of 186 were excluded from analysis, while 82 patients were included in the nafcillin group and 75 patients in the cefazolin group. An additional 8 patients were excluded from the clinically evaluable population due to AKI at the time of beta-lactam initiation (Figure S1). Table 1 highlights the differences in baseline characteristics including male sex (p = 0.003), age (p < 0.001), and history of penicillin allergy documented in chart (p = 0.006). Table S1 provides additional details of selected patient and treatment characteristics.
The primary endpoint, incidence of AKI, was 26/81 (32%) versus 9/68 (13%) (p = 0.007), in the nafcillin and cefazolin arms, respectively. AKI with 50% increase in baseline creatinine was experienced by 21/81 (25.9%) in the nafcillin and 7/68 (10.3%) in the cefazolin arms (p = 0.015). In multivariable logistic regression, nafcillin was an independent predictor of AKI [adj odds ratio (OR) = 2.74; 95% (CI), 1.1–6.6] after adjusting for endocarditis and stay in an intensive care unit (Table 2). Additional variables thought to be clinically relevant but were not different at baseline between the two groups included: vancomycin >4 g per day prior to initial beta-lactam, concomitant use of >1 additional nephrotoxin (vasopressors, loop diuretics, aminoglycosides or vancomycin), and PITT bacteremia score ≥4. The probability of experiencing AKI increased with nafcillin compared with cefazolin as the duration of treatment increased (p = 0.011) and is demonstrated by Fig. 1. Nine patients (11%) initially treated with nafcillin were switched to cefazolin therapy after 72 h due to nephrotoxicity, compared with no switches in the cefazolin arm (p = 0.005).
The median duration of vancomycin therapy prior to switch to initial beta-lactam was 3 days for each group (p = 0.783). Treatment failure was identified in 17.6% of patients treated with cefazolin and 13.6% for nafcillin (p = 0.494). Treatment success was determined in 82.4% and 86.4% in the cefazolin and nafcillin arms, respectively. All-cause 30 day mortality was rare and was not different between the two treatment groups (6% for cefazolin vs. 5% for nafcillin, p = 0.538). Clinical failure secondary to recurrent infection was determined in 1 of 68 (1.5%) for cefazolin and 2 of 81 (2.5%) in the nafcillin arm (p = 0.666). In a subgroup analysis of endocarditis patients, overall clinical failure was experience in 4/11 (36.4%) of cefazolin patients and 4/22 (18.2%) of nafcillin patients. The incidence of additional adverse events for cefazolin versus nafcillin were: Clostridium difficile 0% versus 6.3% (p = 0.036), neutropenia 2.5% versus 2.9% (p = 0.869), increased liver function tests to greater than 3 times the upper limit of normal 2% versus 9.9% (p = 0.032) and drug rash 4.4% versus 4.9% (p = 0.88).
Overall length of stay was similar between the two groups: 12 days [10–14] for cefazolin and 14 days, [11–15] for nafcillin (p = 0.467). Patients with endocarditis had a longer duration of bacteremia, 3 days [2–5], compared with those patients who did not have endocarditis, 2 days [2, 3] (p = 0.028). For patients who experienced AKI compared with those who did not have AKI, they were more likely to have an increased length of stay in the intensive care unit 14 versus 10 days respectively. When comparing drug costs alone for patients who experienced AKI, they were $2271 for nafcillin versus $75 for cefazolin. The decision tree is described in Fig. 2, which shows that, when cefazolin is used for MSSA bacteremia, there is a 96% chance of clinical success and an estimated total cost of $16,059 compared with 83% and $28,195, respectively, for nafcillin. The incremental cost-effectiveness ratio could not be calculated as cefazolin was dominant.
Discussion
Since anti-staphylococcal penicillins and first-generation cephalosporin agents are recommended as first-line treatment options for MSSA bacteremia, it is important to identify differences among the agents in terms of their safety and cost of therapy. Previously, when nafcillin and cefazolin were evaluated in the outpatient setting, the incidence of renal impairment was found in 11.4% in nafcillin and 3.4% of cefazolin patients [10]. In the inpatient setting, an increase in serum creatinine levels ≥1.5 times baseline occurred more commonly with nafcillin than with oxacillin (18% vs. 6%; p = 0.03). Additionally, nafcillin had a higher incidence of discontinuation due to nephrotoxicity compared to oxacillin (9.4% vs. 0%, p = 0.007) [11]. The present study confirms this prior literature and suggests that first-generation cephalosporins appear to have a more favorable side effect profile due to less nephrotoxicity compared with nafcillin therapy, and are more cost-effective. The statistical association was consistently observed using a nephrotoxicity definition of 0.3 mg/dL increase within 48 h and using any 50% increase. We identified nephrotoxicity in 32% (nafcillin) and 13% (cefazolin) of patients using the more sensitive definition, and 26% and 10%, respectively, using a more traditional definition. This incidence of AKI is greater than previous studies. This may be due to differences in the patient populations studied, including the severity of illness and proportion of patients with endocarditis in our study.
Another patient focused outcome that we evaluated in our study was length of stay. The length of stay was numerically higher in patients on nafcillin at 14 days compared to 12 days for cefazolin; however, as this was not our primary endpoint, this study was not powered to detect a difference. Endocarditis as the source of infection, comorbidities, and development of acute kidney injury are among the many factors that could contribute to an increased length of stay. Data from this study may help to improve safety monitoring of beta-lactams for MSSA bacteremia. Early identification of acute kidney injury may ultimately reduce length of stay.
Although there is a lower average wholesale price for cefazolin versus oxacillin treatment ($150 per day) or nafcillin ($166 per day), drug acquisition cost is a minor component on overall cost burden. Patients who experienced AKI in our study had a longer length of stay in the intensive care unit, contributing to a larger difference in overall costs between the agents. After adjusting for a diagnosis of endocarditis and ICU admission, nafcillin was still independently associated with AKI. Use of cefazolin for patients with MSSA endocarditis is somewhat controversial and generally reserved for patients with penicillin allergy [14]. This recommendation stems from evidence suggesting clinical failures due to an inoculum effect [15]. Our subgroup of 33 patients with endocarditis support this recommendation, as 4/11 (36.4%) of cefazolin patients and 4/22 (18.2%) of nafcillin patients experienced clinical failure. However, 3/11 (27.3%) of cefazolin patients and 11/22 (50%) of nafcillin patients with endocarditis experienced AKI.
This study had several limitations, including the retrospective design and single health-system. The primary endpoint of AKI was derived from previous literature but may be too sensitive. However, the improved safety with cefazolin was also demonstrated with a more conservative definition of 50% increase from baseline. Some selection bias is likely present, as patients who experienced AKI prior to initiation of beta-lactam therapy were more likely to receive cefazolin. Urine eosinophils were only collected from three of the patients who experienced AKI and there were no kidney biopsies obtained to classify the type of AKI that occurred. Therefore, there is no way of knowing if the AKI was acute interstitial nephritis or due to other causes. Beta-lactamase typing was not performed, as it is not a standard of care and was not available at our institution. No differences were found in concomitant nephrotoxins between the two groups; however, not all nephrotoxins were evaluated. Despite these limitations, the existing body of literature suggests that cefazolin may be the preferred beta-lactam in patients with MSSA bacteremia without central nervous system involvement. In patients with endocarditis, a careful risk–benefit evaluation is indicated and future studies should be carried out to evaluate these differences prospectively.
Conclusion
In conclusion, risk of nephrotoxicity is increased with nafcillin compared with cefazolin. Considering the safety profile and acquisition cost of cefazolin, it may be considered as a preferred therapy to nafcillin for patients with MSSA bacteremia without endocarditis or central nervous system involvement.
References
Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Internal Med. 2002;162(19):2229–35.
Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330–41.
Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–7.
Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
Lee S, Choe PG, Song K, et al. Is cefazolin inferior to nafcillin for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother. 2011;55:5122–6.
Paul M, Zemer-Wassercug N, Talker O, et al. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteremis? Clin Microbiol Infect. 2011;17:1581–6.
Pollet S, Baxi SM, Rutherford GW, Doernberg SB, Bacchetti P, Chambersa HF. Cefazolin versus nafcillin for methicillin-sensitive Staphylococcus aureus bloodstream infection in a California tertiary medical center. Antimicrob Agents Chemother. 2016;60:4684–9.
Rao SN, Rhodes NJ, Lee BJ, et al. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2015;59:5232–8.
Bai A, Showler A, Burry L, et al. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteremia: results from a multicenter cohort study. J Antimicrob Chemother. 2015;70:1539–49.
Youngster I, Shenoy ES, Hooper DC, Nelson SB. Comparative evaluation of the tolerability of cefazolin and nafcillin for the treatment of methicillin-susceptible Staphyloccocus aureus infections in the outpatient setting. Clin Infect Dis. 2014;59(3):369–75.
Viehman JA, Oleksiuk LM, Sheridan KR, et al. Adverse events lead to drug discontinuation more commonly among patients who receive nafcillin than among those who receive oxacillin. Antimicrob Agents Chemother. 2016;60(5):3090–309.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Valour F. Antimicrobial-related severe adverse events during treatment of bone and joint infection due to methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58(2):746–55.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications a scientific statement for healthcare professionals from the American Heart Association. Endorsed by the Infectious Diseases Society of America. Circulation. 2015;132:17–8.
Nannini EC, Stryjewski ME, Singh KV, et al. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphyloccocus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother. 2009;53(8):3437–41.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Dr. Davis has received grant funding from Merck and Allergan and served as an advisory board member and speakers bureau participant for Allergan. Dr. Zervos has received research grant funding from Rempex, Paratek, Merck, Cempra, Melinta, and Achaogen. Drs. Kenney and Flynt do not have any disclosures.
Compliance with Ethics Guidelines
This study was approved by the institutional review board at Henry Ford Health System. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/E197F06020A00B5D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Flynt, L.K., Kenney, R.M., Zervos, M.J. et al. The Safety and Economic Impact of Cefazolin versus Nafcillin for the Treatment of Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections. Infect Dis Ther 6, 225–231 (2017). https://doi.org/10.1007/s40121-017-0148-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-017-0148-z