Abstract
Introduction
Stress is a complex life occurrence essential for survival and goal achievement but can be damaging in excess. Because of the high prevalence of stress in North America, a safe supplement that effectively reduces stress is in demand. The objective of this study was to investigate the efficacy and safety of AlphaWave® l-Theanine on whole-scalp and frontal alpha power, midline theta power, and salivary cortisol in healthy, moderately stressed adults.
Methods
This was a randomized, triple-blind, placebo-controlled, crossover study that consisted of two study periods with a 7-day washout. A single dose of AlphaWave® l-Theanine (200 mg) or placebo was administered. To induce stress, a mental arithmetic test (MAT) was administered before and after the dose. Electroencephalogram, salivary cortisol, blood pressure, heart rate, self-reported stress, adverse events, clinical chemistry, and hematology were assessed to evaluate efficacy and safety.
Results
Increases in heart rate, blood pressure, and self-reported stress and state anxiety indicated that participants experienced stress during the MAT. AlphaWave® l-Theanine led to a greater increase in frontal region and whole-scalp alpha power 3 h post-dose compared to placebo (p ≤ 0.050). Within groups, there were increases in alpha power, at 3 h with AlphaWave® l-Theanine, over the whole recording and during the eyes-open portions (p ≤ 0.048) of the alpha task. The changes in alpha wave activity are supported by greater decreases in salivary cortisol 1 h post-dose (p < 0.001) with AlphaWave® l-Theanine compared to placebo.
Conclusion
This study was conducted during the SARS-CoV-2 pandemic, which has had a rapid and significant effect on both physical and mental health around the world. A single dose of AlphaWave® l-Theanine significantly increased frontal region alpha power compared to placebo in response to an acute stress challenge. These changes are indicative of relaxation in the brain and suggest a calming response. AlphaWave® l-Theanine was found to be safe and well tolerated by participants.
Trial Registration
ClinicalTrials.gov identifier NCT04706494.
Plain Language Summary
Stress is a complex part of life that is essential for survival and achieving goals. Too much stress, however, can be damaging. There is a high prevalence of stress in North America, creating a demand for a safe and effective supplement to reduce it. This study investigated the effectiveness and safety of AlphaWave® l-Theanine on brainwaves and salivary cortisol in healthy, moderately stressed adults facing an acute stressor. This was a randomized, triple-blind, placebo-controlled, crossover study that consisted of two study periods with a 7-day washout. A single dose of 200 mg of AlphaWave® l-Theanine or placebo was administered before and after a mental arithmetic test to elicit acute stress. Electroencephalogram, salivary cortisol, blood pressure, heart rate, self-reported stress, and safety were assessed to evaluate efficacy and safety. This study was conducted during the coronavirus pandemic, which has had a rapid and significant effect on both physical and mental health around the world. A single dose of AlphaWave® l-Theanine had significant positive effects on brainwaves, salivary cortisol, and self-reported state anxiety compared to the placebo in response to an acute stress challenge. These changes are indicative of relaxation in the brain and suggest a calming response in a moderately stressed but otherwise healthy population. AlphaWave® l-Theanine was found to be safe and well tolerated by participants.
Similar content being viewed by others
Adults facing acute and chronic stressors account for up to three quarters of Americans and can cost the workforce ~ $300 billion annually in lost productivity. |
The objective of this study was to determine the safety and efficacy of AlphaWave® l-Theanine in whole-scalp and frontal alpha power (indicators of relaxation) in moderately stressed adults using an acute stress model. |
We found that a single dose of AlphaWave® l-Theanine led to a greater increase in alpha power 3 h post-dose compared to placebo. |
These data suggest that AlphaWave® l-Theanine reduced stress when moderately stressed but otherwise healthy adults were facing an acute stressor. |
Future work should investigate the safety and efficacy of prolonged l-theanine supplementation in healthy populations to provide insight into application to vulnerable populations diagnosed with stress-related disorders, such as general anxiety disorder. |
Introduction
Seventy-five percent of Americans report having experienced a stress-related outcome such as irritability, changes in appetite or sleep patterns, restlessness, or general negative thoughts within the past month [1, 2]. Common sources of stress include finances, the economy, familial responsibilities, health concerns, and work [1, 2]. Job-related stress alone costs American industries an estimated $300 billion a year due to diminished productivity, job accidents, absenteeism, and turnover. Additionally, health care expenditures are nearly 50% greater for workers experiencing high levels of stress [3]. Prolonged stress exposure increases the risk for various health problems and concerns such as mental health issues, substance abuse, and cardiovascular issues, and can even dampen the immune system, increasing susceptibility to both colds and common infections [4, 5]. Indeed, when healthy young adults are exposed to acute mental (mental arithmetic) or physiological (cold pressor test) stress, there are increases in arterial stiffness that are independent of the increases in heart rate and blood pressure [6]. Additionally, a direct relationship has been demonstrated between the physiological response to acute mental stress and future adverse cardiovascular events [4].
Stress is a complex part of life that is necessary for survival and goal achievement but can be damaging in excess. It was first defined clinically by Hans Selye as the nonspecific response of the body to any demand for change [7]. More recently, stress has been described as a response to an intrinsic or extrinsic stimulus that causes a deviation from homeostasis [5, 8]. Stressful events lead to a series of responses which are an intricate interaction between the central and peripheral nervous systems and the rest of the body [9]. Further, there are individual differences in stress response due to sex differences, genetic uniqueness, age, and environmental factors like urbanicity [9, 10].
The currently available drug treatments for managing the signs and symptoms of stress can result in side effects, prompting the need for a more suitable approach for pursuing a healthy lifestyle. Treatment and management of stress is a complex process involving interactions between individuals and environmental factors [11]. Lifestyle changes, such as exercise and socialization, are commonly encouraged to deal with stress [9]. Adopting new lifestyle routines is challenging and requires time and compliance to be effective. As a result, a safe supplement that effectively reduces stress levels is in high demand. This is especially applicable for otherwise healthy populations, experiencing moderate levels of stress, who may not require pharmacological interventions or diagnoses.
l-Theanine is an amino acid found in green tea leaves and has been shown in rodent models to block the binding of l-glutamic acid to glutamate receptors (RS)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), kainate, and N-methyl-D-aspartate (NMDA) [12]. l-Theanine can pass through the blood–brain barrier and begins decreasing in concentration 5 h after administration [13]. The amino acid increases the secretion of neurotransmitters such as dopamine and serotonin in the central nervous system 30 min after oral administration [14]. l-Theanine intake reduces the heart rate (HR) and salivary immunoglobulin A (sIgA) in response to acute stress, attributable to changes in sympathetic activation signals [15]. It is characterized by its ability to induce relaxation when consumed. Apart from its ability to act as a neurotransmitter and decrease blood pressure (BP), l-theanine can stimulate the generation of alpha brainwaves [16]. Increases in brainwave alpha power is considered to be indicative of relaxation [16, 17]. Frontal region alpha waves are observed during non-rapid eye movement (REM) sleep and meditation [17]. Juneja et al. reported that alpha waves were generated in the occipital and parietal regions of the brain surface of human participants within 40 min of oral l-theanine administration [16]. Kobayashi et al. found that after a single dose of l-theanine, individuals with higher anxiety emitted significantly stronger alpha brainwaves than did individuals with lower anxiety [18], indicating a greater effect. These electroencephalography (EEG) studies have shown that l-theanine had a direct effect on the brain and relaxed the mind without inducing drowsiness [16, 18].
With the reported success that l-theanine has had in promoting relaxation and reducing signs of stress, it is possible that a single-dose administration may have an efficacious role for individuals facing an acute stressor. The objective of this study was to investigate the efficacy of AlphaWave® l-Theanine in response to acute stress as assessed by EEG, salivary cortisol, BP, and HR in a moderately stressed and otherwise healthy adult population. It was hypothesized that in response to a task designed to induce stress, a single dose of AlphaWave® l-Theanine would result in reduced psychological indicators of stress relative to placebo. This study population represents the average North American experiencing the daily stresses of life associated with the activities and responsibilities of work and daily living. Participants were required to have regular sleep–wake cycles, which was important to decrease confounding variables that may have impacted study results. This population self-reported being moderately stressed with no pathologies during the global SARS-CoV-2 pandemic.
Methods
This randomized, triple-blind, placebo-controlled, crossover study was conducted at the KGK Science Inc. clinical site, London, ON, Canada, from 15 June 2020 to 25 September 2020. The Natural and Non-Prescription Health Products Directorate, Health Canada, Ottawa, Ontario, approved this study on 19 December 2019, and research ethics board approval was granted from the Institutional Review Board (IRB) Services, Aurora, Ontario, on 10 January 2020 (Pro00041119). All participants provided written informed consent to participate in the study prior to initiation of any study procedures. The study was conducted in accordance with the Declaration of Helsinki guidelines and its subsequent amendments and was carried out in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP). The trial was registered at ClinicalTrials.gov (NCT04706494).
Study Participants
Participants were men and women aged 19–60 years. All participants had moderate levels of stress as assessed by scores of 14–26 on the Perceived Stress Scale (PSS) [19]. Participants were required to have regular sleep–wake cycles defined as a bedtime between 9:00 pm and 12:00 am and between 7 and 9 h of sleep nightly for at least 3 weeks prior to enrollment. Sleep schedule, diet, supplement use, and exercise were maintained throughout the study, and participants were required to abstain from exercise for 24 h prior to study visits.
Participants were excluded if they had any of the following: self-reported epilepsy, seizures, or clinical diagnosis of anxiety or depression; employment that called for shift work that would disrupt normal circadian rhythm or travel across one or more time zones during the 3 weeks prior to enrollment or during the study; known allergies to the test product; a history of alcohol or drug abuse within 12 months prior to enrollment, reported high alcohol intake defined as > 2 standard drinks/day, use of nicotine or tobacco products within 60 days of enrollment, use of medical cannabis or chronic use of cannabinoid products more than twice a week (occasional use was assessed on a case-by-case basis by the medical director); prescribed use of antiepileptics, antiseizure, sedatives or hypnotics, or antibiotics within 30 days of enrollment, or use of l-theanine, melatonin, valerian root, gamma-aminobutyric acid (GABA), tart cherry, amla (Phyllanthus emblica), ashwagandha (Withania somnifera), Rhodiola, shatavari (Asparagus racemosus), or ginseng supplements within 7–14 days of enrollment; cognitive impairment and/or inability to give informed consent, or chronic health conditions including but not limited to diabetes, hypertension, autoimmune or immune compromised, kidney, liver or thyroid disease, significant gastrointestinal tract disease, blood or bleeding disorder, or any other condition that in the opinion of the medical director may have adversely affected their ability to complete the study or its measures, or posed significant risk to the participant.
Intervention
The investigational product (IP), AlphaWave® l-Theanine (200 mg), and placebo (microcrystalline cellulose, hydroxypropyl methyl cellulose) were provided as capsules by Ethical Naturals, CA, USA. AlphaWave® l-Theanine capsules also contained non-medical ingredients of microcrystalline cellulose, hydroxypropyl methyl cellulose, and silicon dioxide. All study products were identical in appearance (size, color, taste, texture, and packaging) to make them indistinguishable to participants and study personnel. Participants were administered a single capsule with 250 mL of room-temperature water by a trained clinical coordinator. The time of dosing was recorded, and the timing of pre- and post-dose measurements (HR, BP, salivary cortisol, and EEG), questionnaires (State-Trait Anxiety Inventory [STAI] and visual analog scale [VAS]), and mental arithmetic task (MAT) was based on the dose time.
Electroencephalogram (EEG)
EEG data were collected with a GRAEL 4K-EEG amplifier and 32-electrode Quik-Cap (Compumedics Neuroscan, NC, USA). For each EEG recording, participants completed a 12-min alpha task consisting of 3 min with eyes open, 3 min eyes closed, 3 min open, and 3 min closed. During the eyes-open conditions, participants were fixated on an “X” on the wall. EEG data were filtered with a 0.1–50 Hz linear finite impulse filter, and down-sampled from 512 to 256 Hz. Data were cleaned with an independent component analysis and visual inspection to remove artifacts from eye blinks, movement, and noise. The power spectrum was analyzed using fast Fourier transform (FFT) with a 1 s Hamming window with 500 ms overlap. Frequencies of interest were the alpha band (8–12 Hz) and theta band (4–7 Hz).
Outcome Measures
The efficacy of a single dose of AlphaWave® l-Theanine was assessed by EEG, salivary cortisol, BP, HR, the STAI, and a VAS of self-reported stress. Relevant EEG channels were averaged to calculate whole-scalp and frontal region alpha power and midline theta power. Whole-scalp alpha power was averaged across the entire scalp, frontal region alpha power was averaged across the frontal channels only, and midline theta was the average theta power between the Fz and FCz electrodes. Salivary cortisol was collected with the Salimetrics high-sensitivity salivary cortisol enzyme-linked immunoassay (ELISA) kit and analyzed by London Health Sciences Centre core laboratory (London, ON, Canada).
Safety outcomes included post-emergent adverse events, vital signs (BP and HR), clinical chemistry, and hematology. Hematology (white blood cell count with differential, red blood cell [RBC] count, hemoglobin, hematocrit, platelet count , RBC indices [mean corpuscular volume, mean corpuscular hemoglobin (MCH), MCH concentration, red cell distribution width, mean platelet volume]), liver function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin), kidney function (creatinine, electrolytes [Na, K, Cl], and estimated glomerular filtration rate [eGFR]), and random glucose were analyzed from blood drawn at all visits by Dynacare (London, ON, Canada) using standard procedures.
Study Procedures
At screening, eligibility was assessed by the Perceived Stress Scale (PSS), medical history, vital signs, anthropometric measurements, and hematology and clinical chemistry parameters. Eligible participants were dispensed a saliva collection kit and instructed on use. Participants returned for their baseline visit and were randomized to one of two treatment sequences. Immediately upon waking on the morning of clinic days 1 and 2, participants were required to collect a saliva sample according to the instructions provided. In the study clinic, participants were provided a standardized meal 2 h prior to IP administration. One hour prior to IP administration (−1 h), baseline measures were collected for HR, BP, alpha task EEG, salivary cortisol, STAI, and a VAS of stress. Participants then completed a 10-min mental arithmetic test (MAT) with simultaneous HR and BP measures. During the MAT, participants were asked to verbally perform serial subtractions of 7 from 700 while keeping pace with a metronome at 90 beats/min. Incorrect responses were corrected, and participants were asked to start again from 700. With a neutral tone, the clinic staff encouraged the participant to go faster and keep pace with the metronome throughout the test [20]. Following the MAT, saliva was collected, and participants completed the stress VAS and STAI questionnaire (−30 min). Participants were administered a single dose of AlphaWave® l-Theanine or placebo. Forty-five minutes after the single dose, participants completed the MAT with simultaneous HR and BP measures, followed by collection of salivary cortisol, alpha task EEG, stress VAS, and the STAI questionnaire (1 h). At 3 h post-dose, alpha task EEG was collected. At the end of each clinic day, adverse events (AEs), vital signs, hematology, and clinical chemistry parameters were measured for safety outcomes.
After a 7-day washout, participants returned to the clinic for the second period of the crossover and were administered the alternate product. All assessments from the first study period were repeated.
Statistical Analysis
The planned total sample size was 16, with eight randomized to each sequence. With this sample size, it was possible to detect a difference in mean change in salivary cortisol levels of 1.94 mmol/L and a difference in mean change in relative alpha of 4% in a crossover design at an unadjusted overall two-sided alpha of 5%, 80% power, and 20% attrition rate. The sample size estimate for salivary cortisol was based on findings from White et al. evaluating the effects of an l-theanine-based nutrient drink on stress among healthy adults using a similar study design [21]. The difference in mean change in salivary cortisol between l-theanine and placebo was −1.08 mmol/L, with a pooled standard deviation of 2.26 mmol/L. The sample size for the difference in mean change in relative alpha is based on Ahn et al. [22], who evaluated the effectiveness of a wearable EEG device in assessing stress. The pooled standard deviations for relative alpha power (for left and right) before and after an arithmetic test were 3.62% and 3.28%, respectively.
The intention-to-treat population consisted of all participants who received either the IP or placebo and for whom any post-randomization efficacy information was available. Assessment of mean change in outcomes between AlphaWave® l-Theanine versus placebo was conducted using a linear mixed effects model. The model included treatment, sequence, and period as fixed effects and subject as a random effect while controlling for the period-dependent baseline value. A within-group analysis of efficacy endpoints was done using Student’s paired t test or, in the case of intractable non-normality, the Wilcoxon signed-rank test. Within-group analyses were compared to baseline (0 min). HR and BP were measured at 3-min intervals for 15 min. At each time point, these outcomes were compared between groups using repeated-measures linear mixed effects models.
A descriptive analysis was provided for post-emergent AEs which were presented along with the system organ class (SOC), preferred term (PT), and lowest-level term (LLT) by study arm. The number of participants with at least one AE was compared between study arms using Fisher’s exact test. For each group, the change in each blood parameter from screening to end of study was assessed using a paired Student’s t test or Wilcoxon signed-rank test if not normally distributed.
Results
Thirty-six volunteers were screened for the study, and 16 eligible participants were enrolled and randomized to received AlphaWave® l-Theanine—placebo (n = 8) or placebo—AlphaWave® l-Theanine (n = 8) (Fig. 1). One participant in the placebo—AlphaWave® l-Theanine arm dropped out of the study after period 1. Participants’ demographics, anthropometrics, and screening vital signs are presented in Table 1. Participants PSS scores ranged from 14 to 26, indicative of moderate stress.
Electroencephalogram (EEG)
When participants were administered a single dose of AlphaWave® l-Theanine they had a significantly greater increase in frontal region alpha power compared to placebo at 3 h post-dose during the eyes-open portions of the alpha task (p = 0.038) (Fig. 2). Frontal region alpha power at 3 h was significantly greater than pre-dose with AlphaWave® l-Theanine over the whole recording and during the eyes-open sections (p ≤ 0.048) that were not observed when participants took placebo. Average within-group percent changes were 70.6% and 56.1% for the whole and eyes-open portions of the alpha task respectively. There was a trend towards a significant increase at 3 h compared to pre-dose with AlphaWave® l-Theanine in the eyes-closed portions (p = 0.094), with a 51.5% average within-group percent change.
Mean frontal alpha power for AlphaWave® l-Theanine and placebo at pre-dose, 1 h post-dose, and 3 h post-dose in the study population. Values presented are mean ± standard error of mean (SEM); a indicates different from pre-dose within-group p < 0.05, # indicates between-group p < 0.05, α indicates a within-group trend of p < 0.1
There was a significant between-group increase (p = 0.050) in whole-scalp alpha power (Fig. 3) during the eyes-open portion of the EEG alpha task in the AlphaWave® l-Theanine group compared to placebo. Significant within-group increases in whole-scalp alpha power during the EEG alpha task were observed 3 h post-dose compared to pre-dose with a single dose of AlphaWave® l-Theanine over the whole recording and during the eyes-open portions (p ≤ 0.026) that were not observed with placebo. Average within-group percent changes with AlphaWave® l-Theanine were 109.0% and 50.6% for the whole and eyes-open portions of the task, respectively. There was a trend towards a significant increase in whole-scalp alpha power at 3 h with AlphaWave® l-Theanine in the eyes-closed portions (p = 0.081), with 401.1% average within-group percent change.
Mean whole-scalp alpha power AlphaWave® l-Theanine and placebo at pre-dose, 1 h post-dose, and 3 h post-dose in the study population. Values presented are mean ± standard error of the mean (SEM); a indicates different from pre-dose within-group p < 0.05, # indicates between-group p < 0.05, α indicates a trend of p < 0.1 within-group
There were no significant between- or within-group differences in frontal midline theta power.
Salivary Cortisol
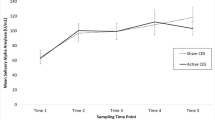
There was a significantly greater decrease in salivary cortisol at 1 h post-dose and following the MAT test, when participants were administered a single dose of AlphaWave® l-Theanine compared to placebo (p < 0.001) (Table 2). Within-group reductions were observed at 1 h compared to −1 h with a single dose of AlphaWave® l-Theanine and placebo, respectively (p ≤ 0.004). The average within-group percent changes were −42.4% and −32.6% when participants took AlphaWave® l-Theanine and placebo, respectively.
Stress Response During MAT: Heart Rate, Blood Pressure, STAI, VAS
A significant stress response was elicited during the MAT test before and after the single dose administration of the IP and placebo. This was evidenced by within-group increases in HR with both AlphaWave® l-Theanine and placebo at 3, 6, and 9 min compared to 0 min during the MAT (p ≤ 0.01) (Fig. 4). Pre-dose, there were between-group differences in HR at 6 and 9 min, where increases compared to 0 min were greater when participants had the placebo. Post-dose, AlphaWave® l-Theanine had greater increases from 0 min to 3 and 6 min (p ≤ 0.024) and placebo at 9 min (p = 0.004). These changes in HR were accompanied by increases in systolic and diastolic BP at several time points from 3 to 9 min (Fig. 5).
Mean heart rate for AlphaWave® l-Theanine and placebo at pre-dose, 1 h post-dose, and 3 h post-dose in the study population. Values presented are mean ± standard error of the mean (SEM); a indicates p < 0.05 within AlphaWave® l-Theanine compared to 0 min, b indicates p < 0.05 within placebo compared to 0 min, # indicates the change from 0 min between-group p < 0.05
Mean systolic (solid) and diastolic (dashed) blood pressure for AlphaWave® l-Theanine and placebo at pre-dose, 1 h post-dose, and 3 h post-dose in the study population. Values presented are mean ± standard error of the mean (SEM); a indicates p < 0.05 within AlphaWave® l-Theanine compared to 0 min, b indicates p < 0.05 within placebo compared to 0 min, # indicates the change from 0 min between-group p < 0.05
There were increases in state anxiety (Table 3) and stress on the VAS (Table 4) during the pre-dose MAT test (p ≤ 0.002) with both products, which were greater prior to the AlphaWave® l-Theanine dose (p < 0.001) relative to placebo. Post-dose there were no significant between- or within-group differences in state anxiety or stress. Trait anxiety was not affected by any product or at any time point.
Safety
All post-emergent AEs were categorized as “unrelated” or “unlikely” to be related to the IP. There were no clinically relevant changes in hematology or clinical chemistry from screening to day 8 in participants enrolled in this study, with the exception of elevated AST and ALT liver enzymes in one participant. The incident finding was classified as unlikely to be related to the product and did not have an impact on study findings. With the exception of this participant, all markers out of laboratory range were deemed not clinically significant by the medical director.
Discussion
This clinical study assessed the efficacy and safety of a single dose of AlphaWave® l-Theanine on whole-scalp and frontal alpha power, midline theta power, and salivary cortisol in moderately stressed and otherwise healthy adults in a population with moderate levels of self-reported stress. The study was conducted during the global SARS-CoV-2 pandemic, at a time when there was an accentuation of the daily stresses of life when many people across the world were experiencing higher than their usual levels of stress. Following a task-induced stress protocol and a single dose of AlphaWave® l-Theanine, healthy adults showed significant increases in whole-scalp and frontal region alpha power in their brain and reductions in salivary cortisol. To our knowledge, this is the first study to investigate the effects of l-theanine in a population of healthy adults reporting moderate levels of stress.
The stress model used in this study was successful in eliciting an acute stress reaction as evidenced by the physiological and subjective indications of stress. The expected physiological changes during a stress test are increases in HR and BP. The study results suggest that participants demonstrated these physiological responses. These physiological changes were confirmed by significant increases in self-reported stress and state anxiety pre-dose. Study findings suggest that the MAT test protocol was effective in inducing an acute, stressful event for the participants in this study and support its use in future clinical trials in the nutraceutical industry.
The effect of the stress test was observed in EEG measured alpha waves through nonsignificant reductions in whole-scalp and frontal alpha power at 1 h post dose. Increases in brainwave alpha power is considered to be reflective of relaxation [16, 17]. Frontal region alpha waves are observed during non-REM sleep and meditation [17]. When participants received the single dose of AlphaWave® l-Theanine, they responded with significantly increased frontal region alpha power during the EEG recording at 3 h. In addition to the improvement in frontal region alpha power, there were significant within-group increases in whole-scalp and frontal alpha power over the full EEG recording and during the higher arousal state, eyes-open portions with AlphaWave® l-Theanine that were not observed when participants consumed placebo. This is significant because there is greater arousal and activation with visual stimulation from eyes open compared to eyes closed, resulting in expected differences in alpha power [23]. Previous studies have reported approximately 40% lower alpha power with eyes open compared to closed [23]. By examining the eyes-open and eyes-closed portions of the alpha task separately, the effects of AlphaWave® l-Theanine in two arousal states were assessed. Participants were exposed to task-induced stress 1 h after consumption of the IP. The results of this study are supported by previous reports of direct effects of l-theanine on alpha waves in the occipital and parietal regions of the brain [14, 16, 18]. The current study investigated the effects of a single 200 mg dose of AlphaWave® l-Theanine. A previous study investigated the effects of lower, 50 mg doses of l-theanine on alpha power and showed increases in a time-dependent manner for 105 min [24]. The current work observed relaxation effects that extended 3 h post-dose, therefore demonstrating the efficacy of AlphaWave® l-Theanine for longer periods of time than previously shown. Future work should continue to investigate dose- and time-dependent effects of AlphaWave® l-Theanine.
Supporting the efficacious changes to whole-scalp and frontal alpha power, there was a significant reduction in salivary cortisol with a single dose of AlphaWave® l-Theanine relative to the placebo, immediately following the stress-inducing task 1 h post-dose. Cortisol plays a crucial role in appropriating the functions that are essential in curbing those that are nonessential or detrimental to the flight-or-fight situation and enhances brain activity. Biologically active cortisol circulates in serum, and salivary cortisol is indicative of circulating levels and can be used as a noninvasive biomarker of stress in clinical research [25, 26]. Cortisol has a diurnal profile, with the onset of the circadian rise occurring in the early morning with the acrophase of the profile upon awakening and the levels decreasing thereafter until reaching a nadir in the late afternoon [27] in those who have a relatively consistent sleep–wake cycle. The enrollment of participants who reported consistent sleep–wake cycles ensured the integrity of the results reported herein. The decrease in the cortisol levels is consistent with what would be expected in both the placebo and the IP group, as it would reflect the cortisol decrease in the diurnal profile. However, there was a greater decrease in cortisol when participants were given a single dose of AlphaWave® l-Theanine versus when they received placebo. Interestingly, a previous study observed that reductions in salivary cortisol were only significant 3 h after a single l-theanine dose and not at the 1-h time points [21]. Studies have indeed shown that an inverse relationship exists between alpha power and cortisol levels [28]. Interestingly, cortisol appears to not always provide the same response when cortical activity is examined. For example, Tops et al. found the opposite response to one of their previous studies when examining the effects of cortisol administration and cortical activity [29]. A likely explanation for this finding is that cortisol is just one of the many factors that impacts alpha power, and it does not provide the full story. Indeed, alpha activity is not only associated with arousal state, but indexes the operation of selective attention [30]. With l-theanine supplementation, alpha amplitude has been demonstrated to be lower in the pre-response and in response to visual and auditory cues compared to placebo [31]. The current study suggests that the l-theanine formulation used in this study showed efficacy as early as 1 h after AlphaWave® l-Theanine administration. Future work is warranted to investigate the long-term effects of a single dose of AlphaWave® l-Theanine, as well as the possibility of using AlphaWave® l-Theanine in longer-term studies in populations that are vulnerable to stress or populations that have received a diagnosis such as general anxiety disorder.
There is long-term potential for l-theanine supplementation for acute and chronic stress management based on the results of this study and previous work [32, 33]. Following the MAT task, there was a significant increase in self-reported state anxiety and no significant changes to trait anxiety. These findings are expected, as state anxiety is reflective of a temporary response to a specific situation, and trait anxiety is a general tendency and personality feature to react to stressful situations in a certain way [34]. Four weeks of supplementation with 200 mg of l-theanine has been shown to improve trait anxiety scores [35], suggesting that continued supplementation with AlphaWave® l-Theanine may have positive effects on trait anxiety as well, which may be an important application in longer-term stress management.
The pandemic has had a rapid and significant effect on both physical and mental health around the world. Systematic reviews and meta-analyses have reported a high prevalence of stress associated with the pandemic in over 23% of the general population in Europe, North America, and Asia [36, 37]. The societal and health burden that may be incurred as a result of additional stress outcomes related to the pandemic warrants safe and efficacious nutraceutical supplements to manage stress, and is a potential application for AlphaWave® l-Theanine requires further attention.
The following limitations to the application of these results should be considered. The population of participants enrolled in this study were those who attested to moderate levels of self-reported stress and did not have a clinical diagnosis of anxiety or depression; therefore, future research is needed to determine the efficacy of AlphaWave® l-Theanine in clinical populations. In this study, participants were administered a 200 mg single dose of AlphaWave® l-Theanine, and the response to an acute stress model was investigated; thus, the efficacy of long-term supplementation with AlphaWave® l-Theanine on moderately stressed healthy adults is currently unknown and warrants further investigation. There have, however, been studies investigating the effects of 8–10 weeks of 250–900 mg/day of l-theanine supplementation in patient populations. In patients with schizophrenia and schizoaffective disorder, 8 weeks of 400 mg/day of l-theanine supplementation resulted in no treatment-related AEs being reported [33]. In an open-label study involving patients with major depressive disorder [38], 8 weeks of 250 mg/day of l-theanine supplementation saw a decrease in high-density lipoprotein (HDL) cholesterol. However, the authors noted that HDL cholesterol was still considered normal (57.6 mg/dL) and well above the lower-limit safety range (40 mg/dL). Finally, in patients with generalized anxiety disorder taking 450 mg/day of l-theanine for 4 weeks, and then approximately half of the patients increasing to 900 mg/day for an additional 4 weeks, there was no difference in the amount or severity of AEs reported between the two l-theanine groups or the placebo group [32]. These studies speak to the safety of long-term use of l-theanine supplementation for various indications. Therefore, future research is warranted in long-term supplementation with l-theanine in the formulation and dosage used in the current study in moderately stressed but otherwise healthy adults.
The current study provides evidence for the safety and tolerability of a single dose of AlphaWave® l-Theanine in healthy adults between the ages of 19 and 60 years. There were no adverse events reported in this study that were classified as possibly related to the study product. This evidence of a good safety profile is an important step towards investigating longer-term AlphaWave® l-Theanine supplementation in vulnerable populations such as those experiencing chronic stress or burnout. It is noteworthy that significant results were obtained with a single dose in an acute stress model and free-living population of participants during a pandemic, thus meriting further research on continued AlphaWave® l-Theanine supplementation. To support the continued use of l-theanine supplementation, l-theanine supplementation is generally well tolerated in humans [13], with no studies reporting toxicity in human or animal models [39]. In clinical populations receiving 400–900 mg/day of l-theanine supplementation for 8 weeks for treatment of general anxiety disorder [32] and schizophrenia [33] did not result in serious adverse events compared to placebo. Additionally, the US Food and Drug Administration states that daily consumption of l-theanine should not be greater than 1200 mg/day [39] and Health Canada suggests 200–250 mg/day with no known contraindications or adverse reactions [40]; therefore, as the dosage investigated in this study is below the FDA limit and within the limits of the Health Canada recommendation, the single dose of 200 mg of AlphaWave® l-Theanine is deemed to be safe and well tolerated.
Conclusions
A single dose of AlphaWave® l-Theanine improved the physiological indicators of acute stress in response to the MAT test compared to the placebo, including frontal region alpha power and salivary cortisol, in moderately stressed but otherwise healthy adults. This is significant, as increases in brainwave alpha power is considered to reflect relaxation in the brain, suggesting a calming effect of AlphaWave® l-Theanine. The randomized, triple-blind, placebo-controlled study design is rigorous and is the gold standard for investigating the efficacy of an investigational product. Further, the crossover study design controls for confounding factors between individual participants by measuring the effects from the single dose of AlphaWave® l-Theanine and placebo in the same participant. A single dose of AlphaWave® l-Theanine was found to be efficacious, safe, and well tolerated, and should be considered as a nutraceutical supplement to manage acute stress.
References
Canada Go. Coping with stress. 2008. https://www.canada.ca/en/health-canada/services/healthy-living/your-health/lifestyles/your-health-mental-health-coping-stress-health-canada-2008.html.
Association AP. Stress in America—paying with our health. 2014.
Stress TAIo. Workplace Stress. 2018.
Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension. 2010;55(4):1026–32.
Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J. 2017;16:1057–72.
Jatoi N-A, Kyvelou S-M, Feely J. The acute effects of mental arithmetic, cold pressor and maximal voluntary contraction on arterial stiffness in young healthy subjects. Artery Res. 2014;8(2):44–50.
Szabo S, Yoshida M, Filakovszky J, Juhasz G. “Stress” is 80 years old: from Hans Selye original paper in 1936 to recent advances in GI ulceration. Curr Pharm Des. 2017;23(27):4029–41.
Focus on stress. Nat Neurosci. 2015;18(10):1343.
McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367–81.
Novais AMS, Roque S, Correia-Neves M, Sousa N. How age, sex and genotype shape the stress response. Neurobiol Stress. 2016;6:44–56.
Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol. 2018;49:146–69.
Kakuda T, Nozawa A, Sugimoto A, Niino H. Inhibition by theanine of binding of [3H]AMPA, [3H]kainate, and [3H]MDL 105,519 to glutamate receptors. Biosci Biotechnol Biochem. 2002;66(12):2683–6.
Adhikary R, Mandal V. l-theanine: a potential multifaceted natural bioactive amide as health supplement. Asian Pac J Trop Biomed. 2017;7(9):842–8.
Yamada T, Terashima T, Wada K, Ueda S, Ito M, Okubo T, et al. Theanine, r-glutamylethylamide, increases neurotransmission concentrations and neurotrophin mRNA levels in the brain during lactation. Life Sci. 2007;81(16):1247–55.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–45.
Juneja LRCD, Okubo T, Nagato Y, Yokogoshi H. l-Theanine—a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol. 1999;10:425.
Lee DJ, Kulubya E, Goldin P, Goodarzi A, Girgis F. Review of the neural oscillations underlying meditation. Front Neurosci. 2018;12:178.
Kobayashi KNY, Aoi N, Juneja LR, Kim M, Yamamoto T, Sugimoto S. Effects of l-Theanine on the release of α-brain waves in human volunteers. Nippon Nogeikagaku Kaishi. 1998;72(2):153–7.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96.
Jern SP, Jern C, Carlsson SG. Short-term reproducibility of a mental arithmetic stress test. Clin Sci (London). 1991;81:593–601.
White D, de Klerk S, Woods W, Gondalia S, Noonan C, Scholey A. Anti-stress, behavioural and magnetoencephalography effects of an l-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2016;8(1):53.
Ahn JW, Ku Y, Kim HC. A novel wearable EEG and ECG recording system for stress assessment. Sensors (Basel). 2019;19(9).
Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118(12):2765–73.
Nobre ARA, Owen GN. l-Theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr. 2008;17:167–8.
Turpeinen U, Hamalainen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013;27(6):795–801.
Blair J, Adaway J, Keevil B, Ross R. Salivary cortisol and cortisone in the clinical setting. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):161–8.
Pilger A, Haslacher H, Meyer BM, Lackner A, Nassan-Agha S, Nistler S, et al. Midday and nadir salivary cortisol appear superior to cortisol awakening response in burnout assessment and monitoring. Sci Rep. 2018;8(1):1–12.
Kamei T, Toriumi Y, Kimura H, Kumano H, Ohno S, Kimura K. Decrease in serum cortisol during yoga exercise is correlated with alpha wave activation. Percept Mot Skills. 2000;90(3):1027–32.
Tops M, van Peer JM, Wester AE, Wijers AA, Korf J. State-dependent regulation of cortical activity by cortisol: an EEG study. Neurosci Lett. 2006;404(1):39–43.
Gomez-Ramirez M, Kelly SP, Montesi JL, Foxe JJ. The effects of l-theanine on alpha-band oscillatory brain activity during a visuo-spatial attention task. Brain Topogr. 2009;22(1):44–51.
Gomez-Ramirez M, Higgins BA, Rycroft JA, Owen GN, Mahoney J, Shpaner M, et al. The deployment of intersensory selective attention: a high-density electrical mapping study of the effects of theanine. Clin Neuropharmacol. 2007;30(1):25–38.
Sarris J, Byrne GJ, Cribb L, Oliver G, Murphy J, Macdonald P, et al. l-Theanine in the adjunctive treatment of generalized anxiety disorder: a double-blind, randomised, placebo-controlled trial. J Psychiatr Res. 2019;110:31–7.
Ritsner MS, Miodownik C, Ratner Y, Shleifer T, Mar M, Pintov L, et al. l-Theanine relieves positive, activation, and anxiety symptoms in patients with schizophrenia and schizoaffective disorder: an 8-week, randomized, double-blind, placebo-controlled, 2-center study. J Clin Psychiatry. 2011;72(1):34–42.
Spielberger CD, et al. Development of the Spanish edition of the State-Trait Anxiety Inventory. Rev Interam Psicol. 1971;5(34):145–58.
Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, et al. Effects of l-Theanine administration on stress-related symptoms and cognitive functions in healthy adults: a randomized controlled trial. Nutrients. 2019;11(10):2362.
Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16(1):1–11.
Cooke JE, Racinea N, Madigan S. Prevalence of posttraumatic and general psychological stress during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2020;292:113347.
Hidese S, Ota M, Wakabayashi C, Noda T, Ozawa H, Okubo T, et al. Effects of chronic l-theanine administration in patients with major depressive disorder: an open-label study. Acta Neuropsychiatr. 2017;29(2):72–9.
Vuong QV, Bowyer MC, Roach PD. L-Theanine: properties, synthesis and isolation from tea. J Sci Food Agric. 2011;91(11):1931–9.
Health Canada. L-Theanine. 2019. http://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq.do?id=7288&lang=eng.
Acknowledgements
The authors wish to thank our volunteers who participated in this study. We wish to thank Dr. Abdul Sulley for the statistical analysis and Dr. Erin Lewis and Dr. Matt Mallette for critical review of the study protocol.
Funding
This study and the journal’s Rapid Service Fee was funded by Ethical Naturals Inc., California, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Conceptualization, ME, LX, NG; methodology, ME, ACM, LX, DCC; formal analysis, ME, ACM; investigation and data curation, ME, DCC.; writing—original draft preparation, ME, ACM; writing—review and editing, ME, ACM, LX.; visualization, ME; supervision, ME, DCC; All authors have read and agreed to the published version of the manuscript.
Disclosures
Lora Xiong is an employee of Ethical Naturals Inc. Malkanthi Evans, Alison C. McDonald, David C. Crowley, and Najla Guthrie are employees of KGK Science.
Compliance with Ethics Guidelines
The trial received research ethics board approval on 10 January 2020 from the Institutional Review Board (IRB) Services, Aurora, Ontario (Pro00041119). All participants provided written informed consent to participate in the study prior to initiation of study procedures. The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) and in accordance with the Declaration of Helsinki guidelines and its subsequent amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Evans, M., McDonald, A.C., Xiong, L. et al. A Randomized, Triple-Blind, Placebo-Controlled, Crossover Study to Investigate the Efficacy of a Single Dose of AlphaWave® l-Theanine on Stress in a Healthy Adult Population. Neurol Ther 10, 1061–1078 (2021). https://doi.org/10.1007/s40120-021-00284-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-021-00284-x