Abstarct
Introduction
Vermicomposting could increase nutrients availability including phosphorus. During vermicomposting, a decomposition of organic substrates leads to the production of several organic acids, such as malonic, fumaric, succinic acids. Microorganisms both in the intestinal organ of the worms and the organic waste have the ability to convert insoluble P into soluble forms. Little information exists about the effects of vermicomposting on rock phosphate (RP) solubilization. Present study was conducted to evaluate the solubilization of powdered RP during vermicomposting.
Results
Vermicomposting and RP application increased NaHCO3-Pi. Rock phosphate application in vermicomposting significantly increased NaHCO3-Po. Vermicomposting significantly increased NaOH-Pi in all of the treatments. RP application and vermicomposting increased HCl-Pi in both organic sources. Generally, vermicomposting increased HCl-Po. Vermicomposting decreased pH but its effect was more evident in the presence of RP. Vermicomposting increased EC in both organic sources.
Conclusion
Present study showed that vermicomposting helps to enhance the transformation of P from RP into various organic or inorganic P forms, which would be readily or moderately available, thus, increase the availability of P from both RPs.
Similar content being viewed by others
Introduction
The high cost of chemical P fertilizer production has generated considerable interest toward direct utilization of rock phosphate in some countries as well as Iran (Besharati et al. 2001). Most of the RPs are reasonably suitable for direct use in acid soils, but has not given satisfactory results in neutral to alkaline soils (Narayanasamy and Biswas 1998). Some methods for improving the efficacy of RP are mixing with elemental sulfur (Mohammady Aria et al. 2010), partial acidulation with nominal amount of acid, dry compaction with water-soluble P fertilizers (Biswas and Narayanasamy 2006) and mixing RP with organic residues, such as compost, manure, and vermicompost (Odongo et al. 2007; Biswas and Narayanasamy 2006).
Vermicompost is an efficient tool to manage the utilization of organic residues (Garg et al. 2006) and increase nutrients availability including phosphorus (Ghosh et al. 1999; Venkatesh and Eevera 2008). During vermicomposting, a decomposition of organic substrates leads to the production of several organic acids such as malonic, fumaric, succinic acids (Epstein 1997), and soluble humic molecules (Atiyeh et al. 2002). Both assimilation and mineralization of phosphorus are microorganisms mediated processes and application of vermicompost increases the rates of these two processes in soil.
Previous studies have revealed the beneficial effect of mixing rock phosphate with vermicompost. Pramanik et al. (2009) repoted that vermicomposts increased (13–26 %) available P of soil after 90 days of incubation. Available P content of rock phosphate-treated soils increased steadily up to 45 days of incubation, but thereafter it gradually decreased forming a parabolic shaped rate curve for P transformation. Initial decrease in P content in vermicompost-treated soils could be avoided or minimized by application of rock phosphate in combination with vermicomposts (Pramanik et al. 2009). Mohammady Aria et al. (2010) observed that vermicompost had a significant effect on the water-soluble phosphates of hard phosphate rock.
Many studies have been conducted about the changes of biochemical properties and nutrients (including P) availability during vermicomposting, but little information exists about the effects of vermicomposting on RP solubilization, and various organic or inorganic P fractions. Present study was conducted to evaluate the solubilization of RP from Esfordi plant during vermicomposting and the influence of RP on pH and EC of the produced vermicompost.
Methods and materials
Preparation of materials
Two organic matters (leaf compost and sheep dung) were used in the present study. Chemical characteristics of organic sources including pH and electrical conductivity (EC) in a 1:2.5 water mixture, loss on ignition by heating at 550 °C for 4 h, organic and inorganic phosphorus soluble in bicarbonate (NaHCO3-Po and NaHCO3-Pi), total P after dry ashing (Kou 1996) and C:P ratio were determined. Water-holding capacity was determined following 24 h drainage of saturated organic materials (Table 1). Moisture content of OM was determined by drying a specified weight under 70 °C for 48 h.
Experimental treatments consisted of a factorial combination of organic matter (compost or sheep dung), powder rock phosphate (Zero, 6 % raw RP, and 2 % modified RP) and earthworms of Eisenia fetida (zero or 20 adult individuals per pot) with three replications in a completely randomized design. Powder of raw and modified RP was obtained from Esfordi phosphate Plant. The modification procedure contains leaching and floatation and includes no acid treatment, thereupon the water-soluble P of both RP was negligible. Physico-chemical characteristics of phosphate materials are presented in Table 2.
Five hundred g of calculated dry organic matter (based on moisture content) was placed in a suitable plastic bag with appropriate amount of RP. After thoroughly mixing, materials were transferred into suitable plastic pots, which contained a three cm layer of rinsed sand beneath. Ten pairs of earthworm species Eisenia fetida were placed on each pot and sprayed gently with water. Pots were kept at 70 % water-holding capacity by weighing each 3 days under lab temperatures. After 2 months organic materials was separated by sieving at suitable moisture content and allowed to air dry.
Chemical analyses
Inorganic and organic P fractions was extracted separately with sodium bicarbonate 0.5 M pH 8.5 after 30 min shaking (NaHCO3-Pi and NaHCO3-Po, respectively), sodium hydroxide 0.1 M after 2 h shaking (NaOH-Pi and NaOH-Po, respectively), hydrochloric acid 0.5 M after 2 h shaking (HCl-Pi and HCl-Po, respectively). Reactive P in the supernatant was determined using the ascorbic acid method at 882 nm (Murphy and Riley 1962) and total P of the supernatants was determined after digestion of a suitable aliquot as described by Fan et al. (1999). Organic P was calculated as the difference between total and reactive P in the extracts. pH and electrical conductivity (EC) were determined in a 1:2.5 OM:water mixture.
Statistical analyses conducted using MSTATC software.
Results and discussion
Phosphorus fractions
Vermicomposting increased NaHCO3-Pi from 145 mg kg−1 in the control of sheep dung to 215 mg kg−1. Application of 6 and 2 % of raw and modified RP increased this fraction to 220 and 228 mg kg−1 but vermicomposting did not considerably change it in the presence of RP. Similar to sheep dung, RP application and vermicomposting increased NaHCO3-Pi of leaf compost. In average, vermicomposting increased NaHCO3-Po by 35 % and the trend was consistent in all of the treatments. Rock phosphate application had a little effect on NaHCO3-Po in the absence of vermicomposting but when vermicomposted significantly increased NaHCO3-Po, i.e., vermicomposting of 2 % modified RP treatments caused 54 and 12 mg kg−1 increase of NaHCO3-Po in sheep dung and leaf compost, respectively (Table 3). Among different P fractions, bicarbonate extractable inorganic and organic P (NaHCO3-Pi and NaHCO3-Po) collectively form the bioavailable or labile P in the soil (Sui et al. 1999). Previous studies have revealed the increase of inorganic available P by vermicomposting. Ghosh et al. (1999) found that saloid-bound P was considerably higher in the earthworm-treated wastes than in the wastes without earthworms, showing that inoculation of earthworms in wastes was useful in increasing the amount of such loosely held and, thereby, easily accessible, forms of P in the organic wastes. Pal Vig et al. (2001) reported that the total available P was significantly higher after vermicomposting than the initial one. In the present study, it was observed that in addition to easily available Pi, easily available Po will be enhanced by vermicomposting.
Sodium hydroxide extractable Pi (NaOH-Pi) in sheep dung was almost threefold of that in leaf compost (Table 4). Sodium hydroxide (NaOH) is mainly used to remove inorganic Al and Fe–P fraction in soils (Adhami et al. 2006) but when used for OM the origin of inorganic P is undefined and in this case it might extract acidic phosphatase. Rock phosphate application had a little effect on this fraction (Table 4), while vermicomposting significantly increased it in all of the treatments. In average, vermicomposting caused 75 % increase of NaOH-Pi, but the effects of the treatments were more evident on NaOH-Po. In average vermicomposting cuased about 100 % increase of NaOH-Po. Application of 2 % modified RP increased NaOH-Po by 30.5 mg kg−1 in sheep dung but the increase was less for leaf compost (Table 4). According to Cross and Schlesinger (1995), soil organic C controls variability of NaOH-Po pool in the soils. The NaOH extracts P mainly from organic components and some amorphous aluminum-containing compounds in soil (Cassagne et al. 2000) that is; the P associated with humic acids or chemisorbed to the surfaces of Fe and Al compounds (Schoenau et al. 1989). The products of microbial decomposition of organic materials might solubilize some of the sorbed or recalcitrant P in soil causing an increase in organic P associated with the humic substances (Reddy et al. 2005). Organic P extracted with NaOH (NaOH-P) is introduced as stable organic P compounds (Fan et al. 1999), though this fraction is not readily available for plants, but the high resistance of this fraction could maintain P availability in an intermediate period. Nonlabile P fractions are thought to be tightly bound to soil particles, and unavailable to plants. The nonoccluded phosphorus, including phosphorus that is extracted with NaOH, is considered to be biologically available over an intermediate time scale (Cross and Schlesinger 1995).
Inorganic P extracted with HCl is attributed to the highly stable Ca–P compounds (i.e., various apatite compounds). This fraction was the highest among various P fractions in all of the treatments and showed the highest increase with RP application. Rock phosphate application increased inorganic P extracted with HCl (HCl-Pi) in both organic sources. The increase of HCl-Pi by the RP addition was almost equal for both organic sources in the absence of vermicomposting. In average, HCl-Pi increased by 209 and 288 mg kg−1 in 2 % modified RP in sheep dung and leaf compost, respectively. In addition, vermicomposting was associated with a great increase of HCl-Pi in both organic sources. On the other hand, vermicomposting in the presence of RP considerably increased HCl-Pi (Table 5). Vermicomposting of leaf compost increased HCl-Pi around 405 mg kg−1 but in the presence of RP the increase was about 598 mg kg−1. In average, vermicomposting increased HCl-Pi by 450 mg kg−1. HCl-Po increased from 960 mg kg−1 in the control of sheep dung to 1,048 mg kg−1 by the addition of 2 % modified RP while the increase was higher in the leaf compost and caused 275 mg kg−1 increase of HCl-Po. Generally, vermicomposting increased HCl-Po in sheep dung and the application of RP caused higher increase of HCl-Po; the trend, which was not observed for leaf compost (Table 5). The HCl-Po is considered to be derived from particulate organic matter which is unavailable in its current form but may become bioavailable after microbial decomposition (Tiessen and Moir 1993).
When organic matter are passed through gut of earthworms, some phosphorous being converted with more availability to plants (Lee 1985), which might be the cause for the increase in available phosphorous content of the vermicompost in the present study. The increase in phosphorus is due to both direct action of worm gut enzymes and indirectly by stimulation of the microflora (Lee 1985).
pH and EC
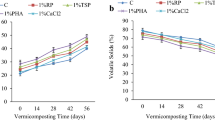
Changes of pH by RP application was low and not consistent for the both organic sources (i.e., RP application decreased pH of sheep dung slightly but not in leaf compost). Vermicomposting decreased pH but its effect was more pronounced in the presence of RP. Vermicomposting decreased pH of sheep dung from 8.82 in control to 8.62 in the absence of RP, but decreased to 7.96 and 7.82 in the presence of 6 and 2 % of raw and modified RP, respectively. Similar results were observed in leaf compost (Fig. 1). This is in accordance with the results of Atiyeh et al. (2000) and Venkatesh and Eevera (2008) and Raphael and Velmourougane (2011). pH decrease may be due to the accumulation of organic acids from microbial metabolism or from the production of fulvic and humic acids during decomposition (Albanell et al. 1988).
Vermicomposting caused the highest changes of EC in both organic sources and its effect seemed to be independent of presence or type of RP. In average, the EC of sheep dung and leaf compost were 4.69 and 2.18 dS m−1 and increased to 5.61 and 2.68 by vermicomposting, respectively (Fig. 2). RP application or type did not cause considerable and consistent changes of EC. The electrical conductivity reflects the salinity of an organic amendment. The increase of EC during vermicomposting is also reported by others (Venkatesh and Eevera 2008; Pal Vig et al. 2001). The increase in EC could be due to loss of organic matter and release of different mineral salts in available forms, such as phosphate, ammonium, potassium, etc. (Venkatesh and Eevera 2008; Pal Vig et al. 2001; Kaviraj and Sharma 2003).
Conclusion
Present study showed that vermicomposting helps to enhance the transformation of P from RP into various organic or inorganic P forms, which would be readily or moderately available. Thus, increase the availability of P from both RPs. RPs application helped the decrease of pH through vermicomposting which would cause higher nutrients availability. EC increased by vermicomposting but RP addition had a little effect on it.
References
Adhami E, Maftoun M, Ronaghi A, Karimian N, Yasrebi J, Assad MT (2006) Inorganic phosphorus fractionation of highly calcareous soils. Commun Soil Sci Plant Anal 37:1877–1888
Albanell E, Plaixats J, Cabrero T (1988) Chemical changes during vermicomposting (Eisenia fetida) of cow manure mixed with cotton industrial wastes. Biol Fert Soils 6:266–269
Atiyeh RM, Domínguez J, Subler S, Edwards CA (2000) Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 44:709–724
Atiyeh RM, Lee S, Edwards CA, Arancon NQ, Metzger JD (2002) The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour Technol 84:7–14
Besharati H, Noorgholipour F, Malakouti MJ, Khavazi K, Lotfollahi M, Ardakani MS (2001) Direct application of phosphate rock to Iran calcareous soils. Paper presented at the international meeting on direct application of phosphate rock and related appropriate technology, Kuala Lumpur, 16–20 July, 2001
Biswas DR, Narayanasamy G (1998) Direct and residual effectiveness of partially acidulated P fertilizers in a cowpea-wheat cropping system. J Indian Soc Soil Sci 46:406–411
Biswas DR, Narayanasamy G (2006) Rock phosphate enriched compost: an approach to improve low-grade Indian rock phosphate. Bioresour Technol 97:2243–2251
Cassagne N, Remaury M, Gauquelin T, Fabre A (2000) Forms and profile distribution of soil phosphorus in alpine Inceptisols and Spodosols (Pyrenees, France). Geoderma 95:161–172
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214
Epstein E (1997) The Science of composting. Technomic Publishing Co. Inc., Lancaster
Fan Y, Xiong H, Li S (1999) Some improvements of the fractionation method of organic phosphorus in calcareous soils. Geoderma 93:195–206
Garg P, Gupta A, Satya S (2006) Vermicomposting of different types of waste using Eisenia foetida: a comparative study. Bioresour Technol 97:391–395
Ghosh M, Chattopadhyay GN, Baral K (1999) Transformation of phosphorus during vermicomposting. Bioresour Technol 69:149–154
Kaviraj, Sharma S (2003) Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour Technol 90:169–173
Lee KE (1985) Earthworms, their ecology and relationships with soil and land use. Academic Press, Sydney
Mohammady Aria M, Lakzian A, Haghnia GH, Berenji AR, Besharati H, Fotovat A (2010) Effect of Thiobacillus, sulfur, and vermicompost on the water-soluble phosphorus of hard rock phosphate. Bioresour Technol 101:551–554
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Narayanasamy G, Biswas DR (1998) Phosphate rocks of India—potentialities and constraints. Fert News 43(21–28):31–32
Odongo NE, Hyoung-Ho K, Choi Hee-chul, Van Straaten P, McBride BW, Romney DL (2007) Improving rock phosphate availability through feeding, mixing and processing with composting manure. Bioresour Technol 98:2911–2918
Pal Vig A, Singh J, Wani SH, Dhaliwa SS (2001) Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour Technol 102:7941–7945
Pramanik P, Bhattacharya S, Bhattacharyya P, Banik P (2009) Phosphorous solubilization from rock phosphate in presence of vermicomposts in Aqualfs. Geoderma 152:16–22
Raphael K, Velmourougane K (2011) Chemical and microbiological changes during vermicomposting of coffee pulp using exotic (Eudrilus eugeniae) and native earthworm (Perionyx ceylanesis) species. Biodegradation 22:497–507
Reddy KR, Wetzel RG, Kadlec RH (2005) Biogeochemistry of phosphorus in wetlands. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment. Agron. Monogr. 46. ASA, CSSA, and SSSA, Madison WI
Schoenau JJ, Stewart JWB, Bettany JR (1989) Forms and cycling of phosphorus in prairie and boreal forest soils. Biogeochem 8:223–237
Sui Y, Thompson ML, Shang C (1999) Fractionation of phosphorus in a Mollisol amended with biosolids. Soil Sci Soc Am J 63:1174–1180
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Can Soc Soil Sci, Lewis Publishers, Boca Raton
Venkatesh RM, Eevera T (2008) Mass Reduction and recovery of nutrients through vermicomposting of fly ash. Appl Ecol Environ Res 6:77–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Adhami, E., Hosseini, S. & Owliaie, H. Forms of phosphorus of vermicompost produced from leaf compost and sheep dung enriched with rock phosphate. Int J Recycl Org Waste Agricult 3, 5 (2014). https://doi.org/10.1007/s40093-014-0068-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40093-014-0068-9