Abstract
Conversion of different agricultural residues including almond shell, walnut shell, barley straw, canola stalk, rice straw and wheat straw to hydrogen-rich gas was performed via gasification in supercritical water media in a determined condition. Elemental characterization was performed using CHNSO analyzer. Besides, cellulose and lignin contents in biomass structure were determined according to TAPPI test methods T264cm-97 and T222om-02, respectively. The correlations between the yields of the product gas components with C/H/O ratio in the initial forms of used feedstocks were investigated. In addition, the relation between the components of biomass structure with the yields of main gaseous products was also studied for each feedstock. The maximum hydrogen yield of 8.38 mmol/g was observed for barley straw which has the highest H percentage of 6.5 wt%. Canola stalk with the highest C/H ratio showed the highest total gas yield of 25.3 mmol/g. Canola stalk with highest amount of cellulose and hemicellulose of 67.42 % had the highest CGE of 45 % and barley straw had the highest HGE of 20 %. Higher C/H value and lower oxygen percentage in the initial form of feedstocks resulted in higher total gas and CO2 yields and lower hydrogen yields. Lignin content in the initial form of the feedstocks was inversely proportional with total gas yields whereas cellulose content showed a straight relation with the total gas yield.
Similar content being viewed by others
Introduction
Employing renewable energies is one of the key strategies for reaching energy security and controlling global warming. Combustion of fossil fuels follows with many environmental disasters. Great amount of research and investments are done for obtaining energy from renewable resources [1]. Biomass is known as the main resource for bioenergy production [2, 3]. It has carbon- and hydrogen-rich nature obtained from CO2 and H2O available in atmosphere [4]. The conversion of biomass is environmentally friendly and sustainable because it is a carbon neutral resource [5, 6]. Currently, near 10 % of world energy consumption is supplied by biomass [7]. Lignocellulosic biomass is the most abundant inedible biomass on earth which is mainly found in agricultural residues. This type of biomass consists of lignin, cellulose and hemicellulose which are degradable into useful fuels and chemicals by novel processing methods [8, 9]. Among the products of biomass conversion, hydrogen as a key energy carrier and versatile and environmental friendly fuel for the future has attracted extensive attention [10, 11]. Hydrogen has the highest energy density among other conventional fuels with LHV of 122 kJ/kg [12]. Also it is combusted, very clean and has zero emission with the only product of water when it is used in fuel cells [13]. However, hydrogen can be obtained from many resources which are mentioned in Fig. 1 [14]. Currently, most of the produced hydrogen is obtained from reforming of fossil fuels but sustainable hydrogen which is produced from renewable resources can be the sufficient objective for reaching sustainable energy development and energy security [15]. As presented in Fig. 1, biomass-based hydrogen production has two main routes: thermochemical method which is the conversion of biomass using heat, and biochemical method which is the conversion of biomass with living organisms [16–18].
Gasification in supercritical water media (T > 374 °C, P > 22.1 MPa) is a novel method for conversion of lignocellulosic feed stocks into gaseous product which mainly consists of CO, CO2, H2 and CH4 [19]. This method has many advantages compared with conventional gasification technologies. Energy-intensive drying step can be avoided and wet biomass can be directly used [20]. Furthermore, water in its supercritical condition has low density and dielectric constant. Consequently, it changes from polar solvent into non-polar solvent and organic compound can be easily solved in it [21]. Many researchers have investigated supercritical water gasification of real biomass and its organic compounds. Lu et al. investigated the gasification of many agricultural residues in 25 MPa and 650 °C. It was seen that wheat straw, sorghum stalk and corn cub had higher gasification efficiencies with lower amounts of lignin compared with others [22, 23]. Madenoglu et al. gasified tobacco stalk and cotton stalk in supercritical water media using stainless steel batch reactor. According to elemental and structural analysis, they reported that tobacco stalk with higher C/H ratio and lower amount of lignin was better gasified [24]. In another experiment, Madenoglu et al. investigated the SCWG of hard-nut shells. Almond shell with lower lignin content was better gasified than walnut shell and hazelnut shell with higher lignin content [25].
In this study, the gasification of various agricultural residues including almond shell, walnut shell, barley straw, canola straw, rice straw and wheat straw in 440 °C and 25 MPa was performed using a stainless steel batch micro-reactor system. Gas yields and product gas compositions were compared with each other considering their structural and elemental analysis. Gasification efficiency and hydrogen selectivity were also calculated for each experiment. The objective of this study is to show how structural composition and the percentage of C, H and O in elemental analysis affect the product gas yield and its composition during the gasification process. Such comprehensive comparison between the gasification performances of major agricultural wastes of Iran has not been investigated before. This research also indicates the comparative potential of these lignocellulosic feedstocks for further studies and applications.
Reaction setup and experimental outline
Materials
Taking into account the main agricultural products of Iran, six biomasses including almond shell, barley straw, canola stalk, rice straw, walnut shell and wheat straw were chosen for experiments. They were supplied from gardens and agriculture farms around Sari and Sanandaj, located in Mazandaran and Kurdistan province of Iran, respectively. They were washed, dried, grounded and sieved to reach the maximum particle size of 150 μm. The elemental analysis of biomass samples was conducted using a CHNSO analyzer (Vario EL III by Elementar, Germany) for characterization.
Experimental setup
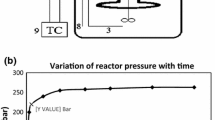
Schematic of the reactor system and experimental setup is indicated in Fig. 2. Stainless steel batch micro-reactor with total volume of 23 mL has been used in this work. 0.05 gram from each feed stock was added to 5 g of deionized water to make a 1 wt% mixture. The mixture was injected into the reactor by a syringe. The reactor was immersed in a molten salt bath containing a mixture of potassium nitrate, sodium nitrate, and sodium nitrite. The molten salt bath temperature was measured using a K-type thermocouple and was remained in 440 °C using a PID temperature controller. Figure 3 indicates the temporal variation of pressure inside the reactor in the determined condition. The corresponding pressure in the reactor at 440 °C after a given reaction time near to 15 min was approximately 250 bars. The reactor was taken out of the molten salt bath and immersed in a water bath for cooling down to room temperature. The final pressure of reaction was measured using a low-pressure gage after opening the high-pressure valve to calculate the amount of the produced gas. Experiments for all six feedstocks were performed three times under the same experimental conditions and reporting data are the averages of repetitive runs [26, 27].
At the end of each experiment, reactor’s free volume, final pressure and temperature were used to calculate the total gas yield. Produced gas composition and the amount of each component was measured using gas chromatograph (Varian 3400 and Teyfgostar-Compact) which used Argon as carrier gas to determine the product gas composition.
Gas samples were taken by tight syringes and injected into gas chromatograph’s column. Gas chromatograph (Varian 3400 and Teyfgostar-Compact) had been equipped with PORAPAK Q-S 80/100 (30 m long, 0.53 mm I.D) column, a methanizer and Flame Ionization Detector (FID). Argon was used as carrier gas and oven temperature program was the following: 40 °C isothermal for 5 min, increase in temperature from 40 to 75 °C in 17.5 min and isothermal at 75 °C for 5 min. The methanizer option enables the FID to detect levels of CO and CO2. During analysis, methanizer is heated to 380 °C with the FID detector body. When the column effluent mixes with the FID hydrogen supply and passes through the methanizer, CO and CO2 are converted to methane. GC was calibrated with standard gas mixture supplied by ROHAM Company in Tehran, Iran. The standard deviation for the results of gas composition was calculated to be ±2 %.
Reaction mechanisms
Reaction mechanisms for main compounds of lignocellulosic biomass have been studied by many researchers [28–30]. Resende et al. studied the non-catalytic hydrothermal gasification of lignin [28]. Azadi et al. studied the SCWG of cellulose, lignin and some other compounds [30]. They observed that cellulose gasified much easier than lignin and showed higher hydrogen yield. However, despite all these research, the mechanism and process of SCWG of real biomass is not completely clear because of the complex structure of lignocellulosic biomass and interactions between components during the process.
Figure 4 presents the schematic pathways for supercritical water gasification of wheat straw as a model compound [31]. Cellulose is an insoluble polymer in the water and consists of glucose subunits. These subunits link with each other by β-1,4-glycosidic bonds [8]. As mentioned in reaction (1), cellulose is hydrolyzed through the rupture of β-1,4-glycosidic linkages to produce glucose [32]. Hemicellulose is a branched amorphous polymer consisting of C5 and C6 sugars linked by various forms of glycosidic bonds. Hemicellulose is hydrolyzed to produce xylose. Connections between cellulose and hemicellulose make networks that stabilize the plant cell wall and lignin covers them. Sugars obtained from cellulose and hemicellulose will be further dehydrated into 5-HMF (5-hydroxymethylfurfural) which in a suitable condition can be converted into acids, alcohols and aldehydes [33]. Lignin is a three-dimensional phenyl propane polymer with ester bond links. It holds cellulose and hemicellulose together in a matrix which forms primary cell wall to prevent plant from damages [8, 34, 35].
Schematic of typical pathway for supercritical water gasification of wheat straw model compounds [31]
Lignin’s hydrolysis in supercritical water, which is followed by dealkylation, is promoting the decomposition of lignin. As mentioned in reaction (3), this process leads to the formation of phenolic compounds such as syringols and guaiacols which will be further form polyphenols [36, 37]. These products are further reformed to syngas by reactions (5) and (6) [28].
In addition to the above reactions, some intermediate reactions can occur to complete the gasification processes successfully which are given below [9, 38].
The reforming reactions produce CO, CO2 and H2, whereas CH4 is produced by methanation via reaction (8). Water–gas shift reaction (WGS) is another significant reaction of SCWG of biomass which is the reforming of CO with water to produce CO2 and H2 via reaction (7). At high CO2 and H2 partial pressures, formation of CO may occur via the reverse water–gas shift reaction [39]. As mentioned, methanation consumes hydrogen and water–gas shift produces hydrogen. So, it is obvious that we should advance the reactions to avoid methanation and accelerate water–gas shift when hydrogen-rich gas is required [40].
Results and discussion
Biomass characterization
The elemental and structural analyses of the biomass particles used in this study are given in Table 1. Lingo-cellulosic biomasses used in this study are mainly made of carbon, oxygen, hydrogen and slight amount of sulfur and nitrogen. In addition, their structures mainly consist of cellulose, hemicellulose and lignin which vary depending on the nature of biomass and the place they have grown. Cellulose and lignin contents were determined according to TAPPI test methods T264cm-97 and T222om-02, respectively [41, 42]. On the other hand, hemicellulose content in the feedstocks was calculated using the standard method [43]. The difference between the amount of lignin, cellulose and hemicellulose depends on the nature of biomass and the place they have grown. This table shows that walnut shell has the highest percentage of lignin and wheat straw contains the highest percentage of cellulose.
Product gas analysis
The gasification efficiencies can be calculated using CHNS analysis and the results obtained by gas chromatograph. Carbon gasification efficiency (CGE) is defined as the ratio of the amount of carbon in the gaseous products of each feedstock to the amount of carbon in the initial feed and Hydrogen gasification efficiency (HGE) is defined as the amount of hydrogen in gaseous product of each feedstock to the amount of hydrogen in the initial feed. Hydrogen selectivity is also calculated via the amounts of hydrogen-containing gaseous products of each biomass. These parameters are mathematically defined as Eqs. (10, 11) [27]:
In this study, experimental conditions including biomass loadings, water loading and temperature were selected according to the previous study which was performed with this reactor by Safari et al. [31]. Figure 5 presents the yields of total gas produced and its main components for all feedstocks. In this figure, the ratio of carbon to hydrogen and the percentage of oxygen in each biomass have been considered. The order of total gas yield is: canola stalk > wheat straw > rice straw > barley straw > almond shell > walnut shell. Except walnut shell, biomasses with higher C/H ratio and lower oxygen content in their initial form were better gasified and had higher total gas yields. Walnut shell has a very high lignin amount in its structure. As shown in Fig. 5, because of the higher lignin content in the structure of this biomass and its complex structure, it resists during hydrolysis and postpones the completion of the process and decomposition in SCWG [44]. In addition, comparing the amount of hydrogen yield, barley straw had the highest yield because of the higher percentage of hydrogen in its initial form and walnut shell had the lowest. For the biomass with nearly same structures, lower C/H ratio resulted in higher hydrogen gas yield and lower CO2 gas yield. Also as mentioned in reaction (8), the yield of CH4 is directly correlated with hydrogen production because of the 3 mol consumption of hydrogen in the methanation process. Consequently, decrease in C/H ratio, increases methanation to some extent.
Figure 6 depicts the gaseous products of different feedstocks in the terms of the lignin and cellulose contents in their structural analysis. It was seen that lower lignin content and higher cellulose content favored higher yields of gases. Lignin is a natural polymer which consists of phenyl propane with ester bond links. It covers cellulose and hemicellulose in a network which forms primary cell walls of plants [8–34]. Lignin content is one of the most influential factors that limits the hydrolysis. But cellulose is made of glucose subunits which linked each other via β-1,4-glycosidic bonds and they can hydrolyse much easier into fermentable sugars [45, 46]. However, the interaction between lignin, cellulose and hemicellulose in SCWG is not clearly specified.
CGE, HGE and hydrogen selectivity for SCWG of different feedstocks are given in Fig. 7. Canola stalk had the highest CGE of 45 % (which is due to higher amount of cellulose and hemicellulose of 67.42 %) and barley straw had the highest HGE of 20 % (which is due to higher weight percentage of hydrogen, 6.5 %). CGE represents the carbon conversion and HGE represents the hydrogen conversion in the process. Higher CGE and HGE mean higher extraction of existing carbon and hydrogen in the biomass, respectively. Hydrogen selectivity means how much of the hydrogen-containing products is in the form of H2. In this study, rice straw had the highest hydrogen selectivity of 6.9. This is due to low methane content in its gaseous products. In addition, barley straw had the lowest hydrogen selectivity. Despite barley’s higher hydrogen yield, its methane yield is much higher and its H2 selectivity is equal to 1.83.
Conclusion
Supercritical water gasification of agricultural wastes including almond shell, barely straw, canola stalk, rice straw, walnut shell and wheat straw were performed using a stainless steel batch micro-reactor at a temperature of 440 °C, pressure of 250 bar, biomass loading of 0.05 g, water loading of 5 g and reaction time of 15 min. Higher C/H ratio and lower oxygen percentage in the biomass results in higher total gas and CO2 yields and lower hydrogen yields. Biomasses with higher amount of cellulose and hemicellulose produced higher amounts of hydrogen gas, whereas presence of lignin in the structure of biomass decreases the hydrogen yield.
References
Wuebbles DJ, Jain AK (2001) Concerns about climate change and the role of fossil fuel use. Fuel Proc Technol 71:99–119
Ballarin A, Vecchiato D, Tempesta T, Marangon F, Troiano S (2011) Biomass energy production in agriculture: a weighted goal programming analysis. Energy Policy 39:1123–1131
Tekin K, Karagöz S, Bektaş S (2014) A review of hydrothermal biomass processing. Renew Sustain Energy Rev 40:673–687
Gerssen-Gondelachs SJ, Saygin D, Wicke B, Patel MK, Faaij APC (2014) Competing uses of biomass: assessment and comparison of the performance of bio-based heat, power, fuels and materials. Renew Sust Energy Rev 40:964–998
Tanksale A, Beltramini JN, Lu GM (2010) A review of catalytic hydrogen production processes from biomass. Renew Sust Energy Rev 14:166–182
Tekin K, Karagöz S (2012) Non-catalytic and catalytic hydrothermal liquefaction of biomass. Res Chem Intermed 39(2):485–498
IEA (2014) Key World Energy Statistics. OECD Publishing, Paris, France
Kobayashi H, Fukuoka A (2013) Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem 15:1740–1763
Safari F, Tavasoli A, Ataei A, Choi JK (2015) Hydrogen and syngas production from gasification of lignocellulosic biomass in supercritical water media. Int J Recycl Org Waste Agric 4:121–125
Najjar YSH (2013) Hydrogen safety: the road toward green technology. Int J Hydrogen Energy 38:10716–10728
Ding N, Azargohar R, Dalai AK, Kozinski JA (2014) Catalytic gasification of cellulose and pinewood to H2 in supercritical water. Fuel 118:416–425
Marone A, Izzo G, Mentuccia L, Massini G, Paganin P, Rosa S, Varrone C, Signorini A (2014) Vegetable waste as substrate and source of suitable microflora for bio-hydrogen production. Renew Energ 68:6–13
Midilli A, Dincer I (2008) Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption. Int J Hydrogen Energy 33:4209–4222
Dincer I, Zamfirescu C (2012) Sustainable hydrogen production options and the role of IAHE. Int J Hydrogen Energy 37:16266–16286
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield—a review. Renew Energy 66:570–579
Kalinci Y, Hepbasli A, Dincer I (2009) Biomass-based hydrogen production: a review and analysis Int. J Hydrogen Energy 34:8799–8817
Balat M (2010) Thermochemical routes for biomass-based hydrogen production. Energy Sources Part A 32:1388–1398
Akalın MK, Karagöz S (2014) Analytical pyrolysis of biomass using gas chromatography coupled to mass spectrometry. TrAC Trends Anal Chem 61:11–16
Mehrani R, Barati M, Tavasoli A, Karimi A (2015) Hydrogen production via supercritical water gasification of bagasse using Ni–Cu/γ-Al2O3 Nano-catalysts. Environ Technol 36:1265–1272
Zhang L, Xu C, Champagne P (2010) Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers Manage 51:969–982
Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew Sust Energy Rev 14:334–343
Lu YJ, Guo LJ, Ji CM, Zhang XM, Hao XH, Yan QH (2006) Hydrogen production by biomass gasification in supercritical water: a parametric study. Int J Hydrogen Energy 31:822–831
Guo LJ, Lu YJ, Zhang XM, Ji CM, Guan Y, Pei AX (2007) Hydrogen production by biomass gasification in supercritical water: a systematic experimental and analytical study. Catal Today 129:275–286
Madenoglu TG, Kurt S, Saglam M, Yuksel M, Gokkaya D, Ballice L (2012) Hydrogen production from some agricultural residues by catalytic subcritical and supercritical water gasification. J Supercrit Fluids 67:22–28
Madenoglu TG, Yildirir E, Saglam M, Yuksel M, Ballice L (2014) Improvement in hydrogen production from hard-shell nut residues by catalytic hydrothermal gasification. J Supercrit Fluids 67:22–28
Afif E, Azadi P, Farnood R (2011) Catalytic hydrothermal gasification of activated sludge. Appl Catal B Environ 105:136–143
Barati M, Babatabar M, Tavasoli A, Dalai AK, Das U (2014) Hydrogen production via supercritical water gasification of bagasse using unpromoted and zinc promoted Ru/γ-Al2O3 nanocatalysts. Fuel Proc Technol 123:140–148
Resende FLP, Fraley SA, Berger MJ, Savage PE (2008) Noncatalytic gasification of lignin in supercritical water. Energy Fuel 22:1328–1334
Zeng J, Singh D, Laskar DD, Chen S (2013) Degradation of native wheat straw lignin by Streptomyces viridosporus T7A. Int J Environ Sci Technol 10:165–174
Azadi P, Khan S, Stroble F, Azadi F, Farnood R (2012) Hydrogen production from cellulose, lignin, bark and model carbohydrates in supercritical water using nickel and ruthenium catalysts. Appl Catal B Environ 117–118:330–338
Safari F, Salimi M, Tavasoli A, Ataei A (2016) Non-catalytic conversion of wheat straw, walnut shell and almond shell into hydrogen rich gas in supercritical water media. Chin J Chem Eng. doi:10.1016/j.cjche.2016.03.002
Boswell JG (2006) The biological decomposition of celluloe. New Phytol 40:20–34
Waldner MH, Vogel F (2005) Renewable production of methane from woody biomass by catalytic hydrothermal gasification. Ind Eng Chem Res 44:4543–4551
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Laadisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 97:673–686
Carrier M, Loppinet-Serani A, Absalon C, Aymonier C, Mench M (2012) Degradation pathways of holocellulose, lignin and α-cellulose from Pteris vittata fronds in sub- and super critical conditions. Biomass Bioenerg 43:65–71
Reddy SN, Nanda S, Dalai AK, Kozinski JA (2014) Supercritical water gasification of biomass for hydrogen production. Int J Hydrogen Energy 39:6912–6926
Brebu M, Vasile C (2010) Thermal degradation of lignin—a review. Cell Chem Technol 44:353–363
Susanti RF, Dianinngrum LW, Yum T, Kim Y, Lee Y, Kim J (2014) High-yield hydrogen production by supercritical water gasification of various feedstocks: alcohols, glucose, glycerol and long-chain alkanes. Chem Eng Res Des 92:1834–1844
Resende FLP, Savage PE (2015) Kinetic model for noncatalytic supercritical water gasification of cellulose and lignin. AIChE J 6:2412–2420
Rashidi M, Tavasoli A (2015) Hydrogen rich gas production via supercritical water gasification of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts. J Supercrit Fluids 98:111–118
TAPPI T 222 om-02 (2002) Acid insoluble lignin in wood and pulp. http://www.tappi/org. Accessed June 2015
TAPPI T 264 cm-97 (1997) Sampling and preparing wood for chemical analysis. http://www.tappi/org. Accessed June 2015
Parsons JL (1963) Holocellulose in wood. Standard Methods Chem Anal 2:1732–1734. http://www.sciencedirect.com/science/article/pii/S0960852413000722
Ding N, Azargohar R, Dalai AK, Kozinski JA (2014) Catalytic gasification of glucose to H2 in supercritical water. Fuel Proc Technol 127:33–40
Yoon SY, Han SH, Shin SJ (2011) The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy 77:19–24
Ju X, Engelhard M, Zhang X (2013) An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol 132:137–145
Acknowledgments
The authors would like to thank the Iran Renewable Energy Organization (SUNA) for their kind support to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Salimi, M., Safari, F., Tavasoli, A. et al. Hydrothermal gasification of different agricultural wastes in supercritical water media for hydrogen production: a comparative study. Int J Ind Chem 7, 277–285 (2016). https://doi.org/10.1007/s40090-016-0091-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-016-0091-y