Abstract
Nanotechnology has been used in many applications and new possibilities are discovered constantly. Recently, a renewed interest has risen in the application of nanotechnology for the upstream petroleum industry, such as exploration, drilling, production and distribution. In particular, adding nanoparticles to fluids may significantly benefit enhanced oil recovery and improve well drilling, such as changing the properties of the fluid, wettability alternation of rocks, advanced drag reduction, strengthening sand consolidation, reducing the interfacial tension and increasing the mobility of the capillary-trapped oil. In this study, we focus on the roles of clay and silica nanoparticles in adsorption process on reservoir rocks. Polymer-flooding schemes for recovering residual oil have been in general less satisfactory due to loss of chemicals by adsorption on reservoir rocks, precipitation, and resultant changes in rheological properties. Adsorption and rheological property changes are mainly determined by the chemical structure of the polymers, surface properties of the rock, composition of the oil and reservoir fluids, the nature of the polymers added and solution conditions such as salinity, pH and temperature. Because this method relies on the adsorption of a polymer layer onto the rock surface, a deeper understanding of the relevant polymer–rock interactions is of primary importance to develop reliable chemical selection rules for field applications. In this paper, the role of nanoparticles in the adsorption of water-soluble polymers onto solid surfaces of carbonate and sandstone is studied. The results obtained by means of static adsorption tests show that the adsorption is dominated by the nanoclay and nanosilica between the polymer molecules and the solid surface. These results also show that lithology, brine concentration and polymer viscosity are critical parameters influencing the adsorption behavior at a rock interface. On the other hand, in this study, the focus is on viscosity, temperature and salinity of solutions of polyacrylamide polymers with different nanoparticle degrees and molecular weight. The adsorption of nanopolymer solution is always higher in carbonated stones than in sandstones, and polymer solutions containing silica nanoparticles have less adsorption based on weight percent than similar samples containing clay. Based on the area of contact for stone, this behavior is the same regarding adsorption.
Similar content being viewed by others
Introduction
Polymer flooding is one of the most successful methods to enhance oil recovery. The polymers that are mainly used in oil fields are water-soluble polyacrylamide (HPAM), xanthan gum (Xc) and associative polymer (AP). Polymer solutions, in contrast to water, exhibit non-Newtonian rheological behaviors, such as shear thinning and shear thickening effects, which lead to different viscosity properties in a reservoir, as compared to those in water flooding. When a polymer solution is injected into a reservoir from an injection well, the fluid velocity, which is related to shear rate, will change from wellbore to in-depth of a reservoir; therefore, the polymer solution viscosity will also change from near wellbore to in-depth of a reservoir correspondingly [21]. The volume of the polymer solution injected may be 50 % PV, depending on the process design [6, 17]. High molecular weight (HMW) polyacrylamide and its derivatives are widely used in oil field applications such as drilling fluids, enhanced oil recovery (EOR) and treatment of oil sand tailings. In these applications, the adsorption characteristics of these polymers are essential since they would affect their applicability and efficiency [1, 9, 11, 12]. Rashidi studied the development and concept of sulfonated polyacrylamide copolymers. Studies were performed on the adsorption and viscosity measurements of the polymer solutions. Parameters that impact these polymers include shear rate, polymer concentration, sulfonate degree, molecular weight, NaCl concentration, divalent ion concentration and temperature. Finally, the results showed that these polymers can be considered as a substitute for hydrolyzed polyacrylamide solution at high salinity and temperature [5, 15].
Moradi performed the rheological measurement of two types of hydrolyzed polyacrylamides with different salinities. These polymer solutions showed both the Newtonian and shear thinning behavior. It was shown that salinity has a major impact on these polymers. The effect of polymer molecular weight and concentration of the polymer solution were analyzed, and the increase in viscosity due to these parameters was reported [13]. With the passing of the era of easy oil and the increasing difficulty of finding new resources, attention of the traditional oil and gas industry has been directed to extract more resources from existing oil fields (enhanced oil recovery) and from the fields exposed to extremely harsh environments using new technologies and solutions. Nanotechnology bears the promise and has received great attention recently from the petroleum industry and there is a general perception that more than a decade’s nanotechnology hype may become nanoreality in the oil field [4, 16], Amanullah 2010).
Nanocomposites produced based on layered silicates such as montmorillonite are of great importance due to their characteristics. Montmorillonite is comprised of many overlapping layers of crystal particles that generally have an average thickness of 0.96 nm and a diameter of 100–200 nm (Fig. 1).
Layer structure of montmorillonite [15]
Polymer adsorption on rock surfaces has been an important issue in EOR applications. EOR applications that use polymer flooding, polymer adsorption or retention in porous media should be avoided. Not all polymer retention is caused by adsorption because some polymer molecules, depending on their size and rheological characteristics, get trapped inside formation pores. It is generally believed that sandstone formations are negatively charged and carbonate formations are positively charged. Clay particles in a formation contain neutral, anionic, and cationic sites. One of the most important properties which made polymers interesting for EOR processes as mentioned earlier was their viscosifying property. Another important parameter which may affect the feasibility study plan of a polymer-flooding project is the retention and adsorption of polymer in porous media. Adsorption and retention may be defined as the interaction between the polymer molecules and the porous medium which leads polymer to be retained or adsorbed [20]. Polymer adsorption/retention mechanisms in porous media are mainly physical interaction, e.g., electrostatic attraction due to the charge differences between the solid surface and polymer or Van der Waals dipole–dipole interactions. Polymer retention is more general, and consists of three main mechanisms: polymer adsorption, mechanical entrapment and hydrodynamic retention as shown in Fig. 2 [20].
General schematic of different polymer retention mechanisms in porous media [15]
Adsorption mechanism which is the major part of retention will be discussed more afterwards, but mechanical entrapment happens when the polymer macromolecules are trapped in narrow pore throat [22]. For polymer adsorption measurement, the mineral sample is soaked in the polymer solution. The difference of polymer concentration before and after mixing with rock sample is measured. The static adsorption onto the mineral surfaces is measured by the depletion method. The unit of retention level (Γ) is the mass of the polymer per unit mass of solid either in mg/g or in g/m2. It is more scientific to measure the surface excess (Γs) which is the mass of polymer per unit surface area of the solid. It should be mentioned that the solid surface is measured by gas adsorption using BET method (Gregg and Sing 1982). Based on the above-mentioned information for static adsorption measurement, the surface excess (Γs) can be calculated as:
where V is the volume of polymer solution with a known concentration ofC1,C2 is the measured concentration of polymer after being adsorbed and A is the total surface area of the adsorbent.
Adsorption is the only mechanism which removes polymer from the solution and results in a significant viscosity reduction at a high level of adsorption. Typically, the adsorption by the bulk static method is much greater than that by dynamic flow conditions [8]. In the static adsorption, measurement flocculation of the mineral particles by polymer bridging is an important parameter which may affect the adsorption. The flocculation is a function of solid–liquid ratio (S/L) [2]. Due to aggregation of mineral particles, increasing the S/L ratio for a polymer solution with known concentration decreases the available surface to the polymer and then decreases total adsorption. On the other side, the very low S/L ratio makes it difficult to measure the adsorbed amount accurately because of the small difference in initial and final concentration [2]. The increase in viscosity, adsorption of polymers onto the reservoir rock presents a limitation for the use of polymers. Adsorption of polyacrylamide (PAM) has been extensively studied [10, 14]. Non-hydrolyzed polyacrylamide adsorption onto kaolinite was studied as a function of electrolyte concentration [10, 14]. With PAM, the adsorption levels were found to be independent of salinity [10], while it was found that adsorption decreases at very low salinities [14, 18, 19].
Experimental
Materials
Fluid properties
-
Brine was prepared from reagent grade NaCl, MgCl2, Na2SO4 and CaCl2 at concentration ranging 20,000.
-
The polymers used were all commercially available, completely water soluble and differed mainly in their concentration (see Table 1). In particular, we tested polyacrylamide that was chemically similar to acrylamide.
Table 1 Chemical composition of bentonite
Local sodium bentonite with a mesh size of 200 and a particle size less than 75 µm was used in this study. The bentonite was produced in Iran and amended with the chemicals shown in Table 1. Oxford-ED2000 XRF and GC-2550TG (Teif Gostar Faraz Company, Iran) were used for all chemical analyses.
Following preparation and analysis of the raw materials, the clay was purified using a 2-inch Hydrocyclon apparatus. To do so, a suspension of 3 wt% clay in distilled water was prepared and then passed through the cyclone at a pressure of 0.15 MPa. This resulted in the removal of impurities and large particles. Montmorillonite particles with a diameter of less than 6 µm were then dried and used in the compatibility process. To ensure compatibility following the dispersion of clay in distilled water, a suspension comprised of clay was prepared and amended with 10, 20, 30, 40 or 50 (wt%) percent of the modifier material “silane”. The mixtures were then heated at 80 °C for 6 h, after which the products were washed with distilled water and dried. The distribution of clay particle sizes was measured before and after purification using a laser particle size analyzer. In addition, clay particles in the colloid state were analyzed by SEM. The intercalation of the samples was evaluated using an X-ray Diffraction [3, 7].
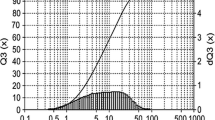
As shown in Figs. 3 and 4, most of the particles in the clay are in the agglomerated form prior to purification. In addition, all of the particles in the clay had diameter of less than 75 µm. Furthermore, 50 % of the particles in the clay were less than 7 µm and 80 % were less than 20 µm in diameter.
Diagram of accumulative distribution of clay particles in feed before purification [7]
SEM image of clay particles in feed before purification [7]
XRD pattern, local pure sample [7]
Diagram of accumulative distribution of clay particles in feed after purification [7]
SEM image of pure clay in magnetite [7]
The results of the XRD analysis revealed that most of the impurities, which included quartz, cristobalite, calcite, gibbsite and feldspar were removed from the clay by the purification process (Fig. 5). In addition, analysis of the particle size distribution (Fig. 6) revealed that most of the particles were less than 5 µm in diameter and that more than 80 % of the particles were less than 2 µm in diameter following purification. Finally, the thickness of the layers in the crystal structure of montmorillonite and the patterns generated by XRD indicate that following purification, the clay comprised crystal layers on the top of each other (Fig. 7).
Rock properties
In this study, the focus is on the polymer adsorption onto the following solids of carbonate and sandstone: (Table 2).
Procedure
Static polymer adsorption experiments were performed to evaluate the adsorption of the different polymer concentrations. These tests were conducted at room temperature by adding sand to the polymer solution and stirring until adsorption was complete. At the end of the test, the samples were reviewed. The amount of polymer retained by the sand was then calculated by difference, after correcting for any sample (Table 3).
Results and discussion
In this section, the results obtained from static adsorption tests and the relationship between polymeric adsorption parameters are introduced.
As researches have shown, nanoparticles have a major role in the case of polymer molecules adsorption on stones. In a classification, one can attribute this dependence to the following:
-
(a)
Polymer viscosity,
-
(b)
Nanoparticles’ concentration,
-
(c)
Stone characteristics,
-
(d)
Reaction temperature,
-
(e)
Durability against stone and solution salinity.
The effects of these factors are being investigated as follows:
-
(i)
Polymer viscosity The current study investigates polymers with different concentrations and from more than 400 samples, the best two results regarding concentration have been selected.
-
(ii)
Concentration of nanoparticles Just like the last case, nanoparticles of silica and clay were investigated.
-
(iii)
Stone characteristics There are various parameters that can be investigated in labs, but by evaluating carbonated and sandstone parameters in this study, different parameters related to reservoir stone are bounded to stone kind.
-
(iv)
Reaction temperature This is one of the parameters affecting viscosity and is supposed as fixed adsorption for this test.
-
(v)
Durability against stone and solution salinity Polymers were evaluated regarding their durability against salinity of different hydrocarbon reservoirs.
All experiments were done within a week and the results showed that the changes after 27,000 s were negligible.
Concentration of nanoparticles
As can be seen from Figs. 8, 9, 10a, the polymer rate is less in sandstone samples. The samples of the same stone with nano-fumed silica have less adsorption than solutions containing nanoclay. Based on contact area of stone, the adsorption shows that the lowest level of polymer is related to sandstones in Fig. 10b and for this test it is related to nano-fumed silica. In this test the concentration of polymer solutions is considered the same.
Considering the contact area of the stone with solution, one can observe that the lowest rate of adsorption for sandstone samples is compared to that of carbonated ones. The lowest levels of adsorption for sand and carbonated stones relate to the samples A24 and B16, respectively, where were tested by the polymer solution I. Transmission electron scanning electron microscope (SEM) was used to determine the morphology (size and shape) of nanoparticles. The SEM images of the prepared nanoclays are shown in the Figs. 11 and 12.
Conclusions
The analysis of adsorption tests supports the following results:
-
The adsorption of nanopolymer solution is higher in carbonated stones than in sandstones that can be attributed to various porosity and cracks of stones.
-
Polymer solutions containing silica nanoparticles have less adsorption based on the weight percent than similar samples containing clay. The same applies regarding adsorption based on the area of contact for stone.
-
We obtained a solution with less viscosity when supplying nanoparticles of both clay and silica in very low rates of polymer concentration that justifies low concentration of polymer.
References
Al-Hashmi, A.R., Luckham, P.F., Heng, J.Y.Y., Al-Maamari, R.S., Zaitoun, A., Al-Sharji, H.H., Al-Wehaibi, T.K.: Adsorption of high-molecular-weight EOR polymers on glass surfaces using AFM and QCM-D. Energy Fuels 27(5), 2437–2444 (2013)
Argillier, J.F., Audibert, A., Lecourtier, J., Moan, M., Rousseau, L.: Solution and adsorption properties of hydrophobically associating water-soluble polyacrylamide. Colloids Surf. 113(3), 247–257 (1996)
Bazgir, S.: Produced for use in polymer nanocomposites. Iran. Rubber Mag. 10, 52 (2006)
Cheraghian, G., Hemmati, M., Masihi, M., Bazgir, S.: An experimental investigation of the enhanced oil recovery and improved performance of drilling fluids using titanium dioxide and fumed silica nanoparticles. J. Nanostructure Chem. 3, 78 (2013)
Delshad, M., Magbagbeola, O.A., Huh, C., Pope, G.A., Tarahhom, F.,: Mechanistic interpretation and utilization of viscoelastic behavior of polymer solutions for improved polymer flood efficiency SPE 113620 presented at the 16th SPE Improved Oil Recovery Symposium, Tulsa, OK, April 21–23, 2008
Green, D.W., Willhite, G.P.: Enhanced Oil Recovery. Society of Petroleum Engineers, Richardson (1998)
Khoeini, M., Bazgir, S., Tamizifar, M., Nemati, A., Arzani, K.: Investigation of the modification process and morphology of organosilane modified nanoclay. Ceramics-Silikáty 53(4), 254–259 (2009)
Lakatos, I., Lakatos-Szabo, J., Toth, J.: Factors influencing polyacrylamide adsorption in porous media and their effect on flow behaviour. Presented at the 3rd International Conference on Surface and Colloid Science, Symposium. On Surface Phenomena in Enhanced Oil Recovery, Stockholm, 29 (1979)
Lakatos, I., Lakatos-Szabo, J.: Investigation of the sorption phenomena of polyacrylamides in porous media under dynamic conditions. Acta Chimica Academiae Scientiarum Hungaricae 105, 57–72 (1980)
Lee, L.T., Rahbari, R., Lecoutier, J., Chateveteau, G.: Adsorption of polyacrylamide on the different faces of kaolinites. J. Colloid Interface Sci. 147(2), 351–357 (1991)
Magbagbeola, O.A.: Quantification of the viscoelastic behavior of high molecular weight polymers used for chemical enhanced oil recovery. MS Thesis, University of Texas At Austin. December, 2008
Md, A., Al-Tahini, A.M.: Nanotechnology—its significance in smart fluid development for oil and gas field application, SPE 126102, 2009
Moradi, H.: Experimental investigation of polymer flow through water- and oil-wet porous media. MS Thesis, University Of Stavanger, July 2011
Pefferkorn, E., Nabzar, L., Varoqui, R.: Polyacrylamide Na-kaolinite interactions: effect of electrolyte concentration on polymer adsorption. J. Colloid Polym. Sci. 265(10), 889–896 (1987)
Rashidi, M.: Physico-chemistry characterization of sulfonated polyacrylamide polymers for use in polymer flooding. Ph.D Dissertation at University Of Bergen, June, 2009
Rassenfoss, S.: 2011 JPT/JPTOnline. 5 November http://www.jptonline.org/index.php?id=1334
Satter, A., Iqbal, G., Buchwalter, J.: Practical Enhanced Reservoir Engineering. PennWell, Tulsa (2008)
Sohn, W.O., Maitin, B.K., Volz, H.: Preconditioning concepts in polymer flooding in high salinity reservoirs: laboratory investigations and case histories. J. SPE Res. Eng. 5(4), 503–507 (1990)
Sorbie, K.S., Johnson, P.A.V., Hubbard, S., Temple, J.: Non-equilibrium effects in the adsorption of polyacrylamide onto sandstone; experimental and modeling study. J. In Situ 13(3), 121–163 (1989)
Sorbie, K.S.: Polymer Improved Oil Recovery. Blackie and Son Ltd, Glasgow (1991)
Sun Y., Saleh., Bai B.: Measurement and Impact Factors of Polymer Rheology in Porous Media, Petroleum Engineering Program, Missouri University of Science and Technology, Rolla, Missouri, USA, March, Rheology, Edited by Dr. Juan De Vicente, ISBN 978-953-51-0187-1. 2012
Willhite, G.P., Dominguez, J.G.: Improved Oil Recovery by Surfactant and Polymer Flooding, Shah, D.O. and Shecter, R.S. (eds), Academic Press Inc., New York (1977)
Acknowledgments
Authors gratefully acknowledge Science and Research Branch of the Islamic Azad University, Khorasan Science and Technology Park and National Iranian South Oil Company for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Cheraghian, G., Khalili Nezhad, S.S., Kamari, M. et al. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int Nano Lett 4, 114 (2014). https://doi.org/10.1007/s40089-014-0114-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40089-014-0114-7