Abstract
In this study, anatase titanium dioxide nanoparticles were successfully prepared by a sol–gel method using two different precursors, titanium isopropoxide and titanium butoxide. Hydrochloric acid or nitric acid was added to adjust the pH of the solution. The sols obtained were dried at 80 °C and calcined at 450 °C for 3 h. The nanostructures were characterised by scanning electron microscopy, FTIR and ultraviolet–visible spectroscopy. The phase transformations were investigated by an X-ray diffractometer. Highly crystalline anatase titania nanoparticles could be obtained through the controlled hydrolysis reaction rate. The sizes of synthesized particles were in the range 5–13 nm, i.e. 9 nm on an average and with a regular shape. The size of nanoparticles was not affected by the choice of precursor. The broad view of the samples prepared using titanium isopropoxide showed film-like structures, whereas the samples prepared using titanium butoxide showed spherical granules. A red shift of 0.13 eV was observed in the band gap in the case of non-spherical particles compared to spherical ones.
Similar content being viewed by others
Introduction
Titanium dioxide (TiO2) exhibits unique characteristics such as low cost, easy handling, non-toxicity and resistance to photochemical and chemical erosion. These advantages make TiO2 a material in solar cells, chemical sensors, hydrogen gas evolution, as pigments, self-cleaning surfaces, and environmental purification applications. Titanium dioxide (TiO2) is one of the most widely used and promising materials in photocatalytic application due to strong oxidizing power of its holes, its redox selectivity, high photostability and easy preparation. An important requirement for high TiO2 photocatalytic efficiency is a large surface area which increases both amount of photon-generated electron hole pairs and surface-adsorbates.
A number of methods [1] such as chemical precipitation, microemulsion, hydrothermal crystallization and sol–gel have been used to enlarge specific surface area of TiO2, mostly, by reducing the particle size down to nanoscale. Sol–gel is one of the most successful techniques for preparing nano-sized metallic oxide materials.
Shipra Mital Gupta and Manoj Tripathi [1] have reviewed the most commonly used solution routes in the synthesis of TiO2. The sol–gel method has been considered to be a promising route for the synthesis of powders for photocatalytic materials. TiO2 nanoparticles have been prepared by a sol–gel method using different precursors such as titanium isopropoxide [2–12], titanium tetrabutoxide [13, 14, 17, 18], titanium tetrachloride [20] and titanium alkoxide [19]. Crystalline TiO2 nanoparticles have also been synthesized by hydrolysis of titanium tetrabutoxide in the presence of acids in NP-5 (Igepal CO-520)–cyclohexane reverse micelle at room temperature [15, 16]. Nanoparticles of TiO2 anatase with mean size in the range 5–10 nm are prepared [20] by precipitation of TiCl4 in aqueous medium in the range 2 ≤ pH ≤ 6. The effect of gelling pH on the properties of the material is studied [8, 11]. The synthesized nanoparticles are found to be of size 7–10 nm [3] or of sizes between 10 and 20 nm [11], 0.1–0.5 μm with an irregular particle shape [8], and about 16 nm [13]. R. Vijayalakshmi and V. Rajendran [4] have found that the nanoparticles prepared via sol–gel route are highly crystalline and have smaller crystallite size (~7 nm) as compared to the one prepared by hydrothermal method (~17 nm) when prepared under the same ambient conditions, viz., temperature, pressure, etc., and keeping all the parameters same viz precursors, mole ratio, solvent, etc. Sanjeevkumar [9] has studied the size-dependent reflective properties of TiO2 nanoparticles and found that the particle size increases from 60 to 150 nm and band gap decreases from 3.42 to 3.14 eV on calcinations of TiO2 powder from 350 to 900 °C. Muneer [6] has investigated the effects of calcination temperatures on the crystalline structure, surface area and photocatalytic activity of TiO2 nanoparticles. Su [17] has found that the crystal size of TiO2 increased from 4 to 35 nm as the calcination temperature is increased from 400 to 700 °C. Effects of the pH value of the solution, H2O2 addition, TiO2 phase composition and recycled TiO2 on the photocatalytic degradation of methyl orange (MeO) in TiO2 suspensions under ultraviolet (UV) illumination are primarily investigated [13]. The photocatalytic activity of titania is investigated [5] from the photodegradation of methylene blue solution under UVC irradiation. Dewi Tristantini and Slamet [12] have used ultraviolet–visible spectroscopy to explore the dispersion and stability of titania nanoparticles in an aqueous media with different types of dispersants. Funda Sayilkan [10] has found that HCl, as a hydrolysis catalyst, plays an important role in the formation of the anatase crystalline form of TiO2 at low temperatures.
Materials and methods
Materials
All reagents used were of analytical grade purity and used as received, with no further purification. Tetraisopropoxide (purity, 97 %), Ti [OC3H7]4 and titanium (IV)-n-butoxide (Ti(OBu)4) (purity 98 %) were used as a starting material and these were supplied by Sigma-Aldrich company. Nitric acid [HNO3] and hydrochloric acid [HCl] were supplied by High Media and ethanol was supplied by Loba. De-ionized water was used to prepare all standard solutions.
Preparation of the samples using sol–gel route
Preparation of samples A, B and C
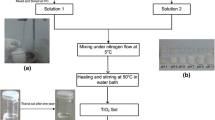
All the reagents used were of analytical grade and no further purification was done before use. The sol–gel synthesized TiO2 was obtained from titanium (IV) isopropoxide (TTIP) was dissolved in absolute ethanol and stirred for 30 min. A mixture of distilled water and hydrochloric acid was added to the solution dropwise with constant stirring. A homogeneous solution was obtained after stirring vigorously for 2 h. A sol was formed. After aging for 24 h, the sol was transformed into gel. In order to obtain nanoparticles, the gel was centrifuged for 15 min at 5,000 rev/s and then dried at 80 °C for 1 h to evaporate water and organic material to the maximum extent. Then the dry gel was sintered at 450 °C for 3 h to obtain the desired TiO2 nanocrystalline.
Preparation of samples D and E
In this work, the precursor solution was a mixture of 5 ml titanium (IV)-n-butoxide (Ti (OBu)4) and 50 ml ethanol. The mixture was stirred for 1 h. The mixture of water, ethanol and HNO3 or HCl solution was then added dropwise into the first mixture. The reaction was performed at room temperature while stirring constantly for 3 h. After aging for 24 h, the sol was transformed into gel. In order to obtain nanoparticles, the gel was dried at 80 °C for 1 h to evaporate water and organic material to the maximum extent. Then the dry gel was sintered at 450 °C for 3 h. to obtain the desired TiO2 nanocrystalline.
Characterisation
The morphology and structure of the particles were investigated using field emission scanning electron microscopy (JEDL JSM-7600F).
The FTIR analysis was done using MAGNA 550, Nicolet Instruments Corporation, USA.
The absorption spectra of the TiO2 samples were measured by UV/Vis spectrophotometer equipped Perkin Elmer (lambda 35) with an integrating sphere.
The prepared nanoparticles were characterized for the crystalline structure using D8 Advance X-ray diffraction meter (Bruker AXS, Germany) at room temperature, operating at 30 kV and 30 mA, using CuKα radiation (λ = 0.15406 nm). The crystal size was calculated by Scherrer’s formula.
Results and discussion
SEM analysis
TiO2 powder samples synthesized based on the different conditions are mentioned in Table 1. The SEM image studies of these samples demonstrate that the size and stability of the sol produced is not affected by the fact that titanium isopropoxide or titanium butoxide is used as a precursor. Also variation in the amount of water does not affect the size of particles synthesized. Moreover whether catalysts used is HCl or HNO3 does not affect the particle size.
Figure 1a–e show SEM micrographs of the TiO2 powders dried at 400 °C for samples A, B, C, D, and E. The TiO2 powder exhibits a homogeneous spherical morphology and a particle size distribution with sizes of 4–16 nm. The particle morphology and particle size distribution show uniformity irrespective of the precursors used for preparing the sample.
But the broad view of the samples A, B, C prepared using titanium isopropoxide shows film like structures whereas the sample D, E prepared using titanium butoxide shows spherical granules.
UV–visible spectra
The absorption spectra of the synthesized TiO2 samples C and D exhibit strong absorptions below 400 nm. See Fig. 2. As reported by K. Madhusudan Reddy [21], the direct transition and not indirect transition, is more favourable in anatase TiO2 nanoparticles. Figure 3a shows the (αEphot)2 versus Ephot plots for the sample C and Fig. 3b shows the (αEphot)2 versus Ephot plots for the sample D. These data result in values of Eg estimated from the α = 0 extrapolation as 3.3 eV for sample C, and 3.43 eV for sample D, which are larger than 3.2 eV Eg for bulk TiO2. This can be explained because the band gap of the semiconductors has been found to be particle size dependent [4]. The band gap increases with decreasing particle size and the absorption edge is shifted to a higher energy (blue shift) with decreasing particle size. Considering the blue shift of the absorption position from the bulk TiO2, the absorption onset of the present samples can be assigned to the direct transition of electrons in the TiO2 nanocrystals. The band gap values validate the crystallite size results according to which smaller crystallite size should have larger band gap (8–13 nm, 3.43 eV). Also the band gap is found to be 3.3 eV for non-spherical morphology (sample C) and it is found to be 3.43 eV for spherical morphology (sample D).
FTIR spectrum analysis
-
In the FT-IR spectrum Fig. 4 of the collected precipitate, a broad peak appearing at 3,100–3,600 cm−1 (precisely at 3,432 cm−1) is assigned to fundamental stretching vibration of O–H hydroxyl groups (free or bonded) [22].
-
The band at 2,920 cm−1 is assigned to C–H vibrations. The C–H can be attributed to the organic residues, which remain in TiO2 even after calcination [2].
-
Also, the sharp peaks centred on 1,621, 1,451, and 1,080 cm−1 can be attributed to C = C (in unsaturated hydrocarbon dehydrated, such as butene, propene from precursors) stretching, –C–H (methyl or methylene) bending and –C–O stretching, respectively [23]. The shoulder observed at 690 cm−1 may have been due to the vibration of the Ti–O–O bond [22].
-
The peak between 800 and 450 cm−1 is assigned to the Ti–O stretching bands [6].
XRD analysis
The phase composition and the crystallite size of the prepared TiO2 samples are evaluated by X-ray diffraction analysis. Figure 5a–d below presents the XRD patterns of calcined TiO2 powder (samples A, B, C, E respectively) at 500 °C temperature for 2 h. The peaks of samples at 500 °C are identified by comparison with JCPDS-84-1286 according to 2θ which confirms an anatase structure at 2θ = 25.4o. It is noteworthy that the diffractograms of the samples do not present any peak assigned to rutile phase (2θ = 27.36°) [6]. XRD pattern (of all samples) reveals that the synthesized titania is composed of anatase as the predominant phase. The reflection from (101), (004), (200), (105), (211) and (204) planes, at 2θ values 25.34°, 38.06°, 48.24°, 54.009°, 55.29°, and 63.09° correspond to anatase phase of TiO2 [9] (JCPDS card #861157). However, according to the XRD results, these particles have their crystallite sizes are nearly 17 and 20 nm.
The average crystallite size is determined using Scherrer’s Formula [2], after carrying slow scan of the powders in the range 24–27° with the step of 0.01° min−1. An estimate of the grain size (G) from the broadening of the main (101) anatase peak is done by using the Scherrer formula below:
where λ is the Cu Kα radiation wavelength and Δ(2θ) is peak width at half-height. The nanocrystallite sizes are found to be 10–25 nm for samples (A, B, C) prepared using titanium isopropoxide and sizes are nearly 60 nm for the sample (E) prepared using titanium butoxide; refer Table 2 below.
Conclusions
-
The particle morphology and particle size distribution have shown uniformity irrespective of the precursors used for preparing the sample.
-
But the broad view of SEM micrographs of the samples prepared using titanium isopropoxide has shown film-like structures, whereas the samples prepared using titanium butoxide have shown spherical granules.
-
The absorption spectra of the synthesized TiO2 sample have exhibited strong absorptions below 400 nm.
-
The band gap values have validated the crystallite size results according to which smaller crystallite size should have larger band gap (8–13 nm, 3.43 eV).
-
Also, for the sample D which showed spherical granules under SEM, the band gap is found to be 3.43 eV, and for the sample C which showed film-like structure under SEM, the band gap is found to be 3.3 eV. Thus, a red shift of 0.13 eV is observed in band gap in case of non-spherical particles compared to spherical ones.
-
XRD pattern (of all samples) has revealed that the synthesized titania is composed of anatase as the predominant phase and the nanocrystallite sizes are found to be 10–25 nm for the samples prepared using titanium isopropoxide and size is found nearly 60 nm for the sample prepared using titanium butoxide .
References
Gupta, S.M., Tripathi, M.: A review on the synthesis of TiO2 nanoparticles by solution route. CEJC 10(2), 279–294 (2012)
Yodyingyong, S., et al.: Physicochemical Properties Of Nanoparticles Titania from Alcohol Burner Calcination. Bull. Chem. Soc. Ethiop 25(2), 263–272 (2011). (ISSN 1011-3924)
Balachandran, K., Venkatesh, R., Shivraj, R.: Synthesis of Nano TiO2-SiO2 composite using sol-gel method: effect on size, surface morphology and thermal stability. IJEST 2(8), 0975–5462 (2010). (e-ISSN, 3895–3700)
Vijayalaxmi, R., Rajendran, V.: Synthesis and characterization of nano-TiO2 via different methods. Archieves Appl Sci Res 4(2), 1183–1190 (2012)
Loryuenyong, V., et al.: Sol gel template synthesis and photolytic behavior of anatase titania Nanoparticles. Sci Asia 38, 301–306 (2012)
Ba-Abbad, M.M., et al.: Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int J Electrochem 7, 4871–4888 (2012)
Yodyingyong, S., et al.: physicochemical properties of nanoparticles titania from alcohol burner calcination. Bull Chem Soc Ethiop 25(2), 263–272 (2011). (ISSN 1011–3924)
Pookmanee, P., Phanichphant, S.: Titanium dioxide powder prepared by a sol-gel method. J Ceram Process 10(2), 167–170 (2009)
Kumar, S., Verma, N.K., Singla, M.L.: Size dependent reflective properties of TiO2 nanoparticles and reflectors made thereof. Dig J Nanomater Bios 7(2), 607–619 (2012)
Sayilkan, F., Asilturk, M., Sayilkan, H.: Characterisation of TiO2 synthesized in alcohol by a sol-gel process: the effects if annealing temperature and acid catalyst. Turk J Chem 29, 697–706 (2005)
Tristantini, D., et al: Modification of TiO2 Nanoparticle with PEG and SiO2 For Anti-fogging and self-cleaning Application. IJET-IJENS. 11(2) (2011)
Safaei-Naeini Y.:Suspension Stability Of Titania Nanoparticles Studied By Uv-Vis Spectroscopy Method. IJMSE. 9(1) (2012)
Yang, H., et al.: Sol–gel synthesis of TiO2 nanoparticles and photocatalytic degradation of methyl orange in aqueous TiO2 suspensions. Elsevier J Alloys Compd 431, 0925–8388 (2006). (ISSN 302–306)
Tang, J., et al.: An Organometallic Synthesis of TiO2 Nanoparticles Nano Letters. Nano Lett 5(3), 543–545 (2005)
Zhang, D., et al.: Formation of crystalline nanosized titania in reverse micelles at room temperature. J Mater Chem 2002(12), 3677–3680 (2002)
Zhang, D., et al.: synthesis of crystalline nanosized titanium dioxide via a reverse micelle method at room temperature. Chinese Chem Lett 14(1), 100–103 (2003)
Su, C., Hong., B.Y., Tseng, C.M.: Sol–gel preparation and photocatalysis of titanium dioxide. Catal Today 96, 119–126 (2004)
Jeffrey, C.S., Tseng, H., Chang, C.: Synthesis of titania-supported copper nanoparticles via refined alkoxide sol-gel process. J NANOPART RES 1388–0764(3), 113–118 (2001). (Journal ISSN)
Karami, A.: Synthesis of TiO2 nano powder by the sol gel method and its use as a photocatalyst. J IRAN CHEM SOC 7, S154–S160 (2010). (ISSN 1735-207X)
Pottier, A., et al.: J Mater Chem 13, 877–882 (2003)
Reddy, K.M., Sunkara, V.M., Reddy, A.R.: Bandgap studies on anatase titanium dioxide nanoparticles. Elsevier Mater Chem Phy 78, 239–324 (2002)
Gaoa, Y., et al.: TiO2 nanoparticles prepared using an aqueous peroxotitanate solution. Ceram Int 30, 1365–1368 (2004)
Chaudhari, V., Shrivastava, A.K., Kumar: On the sol-gel synthesis and characterization of titanium oxide nanoparticles. J Materials Research Society Symp Proc 1. doi:10.1557/opl.2011.759 (2011)
Acknowledgments
The author is grateful to SAIF, Indian Institute of Technology, Mumbai, for their help in characterisation of the samples. Thanks are due to University of Mumbai for funding this project under Minor Research Grant and S. I. E. S. Graduate School of Technology for supporting this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Karkare, M.M. Choice of precursor not affecting the size of anatase TiO2 nanoparticles but affecting morphology under broader view. Int Nano Lett 4, 111 (2014). https://doi.org/10.1007/s40089-014-0111-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40089-014-0111-x