Abstract
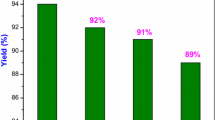
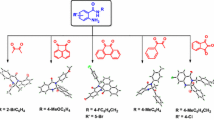
A series of chiral isoselenazolones have been synthesized and characterized by multinuclear (1H, 13C, and 77Se) NMR, mass spectrometry, and single crystal X-ray crystallography. Catalytic reactivity of synthesized chiral isoselenazolones was studied in the enantioselective bromolactonization of alkenoic acids using N-bromosuccinimide as a source of bromine. A series of alkenoic acid underwent bromolactonization successfully in the presence of 10 mol% of chiral isoselenazolone catalyst and excellent yields of bromolactones were obtained with high diastereoselectivity and moderate enantioselectivity. Isoselenazolone catalyst derived from quininamine afforded bromolactones in 82–94 % yield with 1−47 % enantioselectivity while rest of the isoselenazolones derived from simple chiral (S)-phenylethyl, and (S)-naphthylethyl, quinidin, and cinchonidin-amine afforded poor enantioselectivity in the bromolactonization reaction. Furthermore, isoselenazolone having Se–N bond gave best enantioselectivity in the bromolactonization of 5-phenyl-4-pentenoic acid as compared to the isothiazolone having S–N bond. Also isoselenazolone gave best enantioselectivity than the corresponding methyl selenide and diselenide bearing the same quininamine auxiliary in the organoselenium catalyst. Crystal structure and enantioselectivity outcome in bromolactonization on similar isoselenazolone catalysts; quininamine based (intramolecular Se…N distance 3.56 Å, ee 47 %); cinchonidinamine based (Se…N distances 3.80 Å, ee 17 %), and 7-methyl-amide and quininamine based (Se…N distances 3.72 Å, ee 28 %) suggests that intramolecular Se…N interaction could be the crucial parameter for the enantioselectivity outcome of the reaction.







Similar content being viewed by others
References
Back TG (1999) Organoselenium chemistry: a practical approach. Oxford University Press, New York
Wirth T (2010) Organoselenium chemistry: modern developments in organic synthesis (topics in current chemistry). Springer, Berlin
Wirth T (2012) Organoselenium chemistry: synthesis and reactions. Wiley, Weinheim
Wirth T, Kulicke KJ, Fragale G (1996) Chiral diselenides in the total synthesis of (+)-Samin. J Org Chem 61:2686–2689. doi:10.1021/jo952093m
Wirth T, Fragale G (1997) Asymmetric addition reactions with optimized selenium electrophiles. Chem Eur J 3:1894–1902. doi:10.1002/chem.19970031123
Wirth T, Fragale G, Spichty M (1998) Mechanistic course of the asymmetric methoxyselenenylation reaction. J Am Chem Soc 120:3376–3381. doi:10.1021/ja974177d
Wirth T (1999) Chiral selenium compounds in organic synthesis. Tetrahedron 55:1–28. doi:10.1016/S0040-4020(98)00946-6
Wang X, Houk KN, Spichty M, Wirth T (1999) Origin of stereoselectivities in asymmetric alkoxyselenenylations. J Am Chem Soc 121:8567–8576. doi:10.1021/ja990473+
Wirth T (2000) Organoselenium chemistry in stereoselective reactions. Angew Chem Int Ed 39:3740–3749. doi:10.1002/1521-3773(20001103)39:21<3740:AID-ANIE3740>3.0.CO;2-N
Uehlin L, Wirth T (2001) Novel polymer-bound chiral selenium electrophiles. Org Lett 3:2931–2933. doi:10.1021/ol0164435
Uehlin L, Fragale G, Wirth T (2002) New and efficient chiral selenium electrophiles. Chem Eur J 8:1125–1133. doi:10.1002/1521-3765(20020301)8:5<1125:AID-CHEM1125>3.0.CO;2-I
Tiecco M, Testaferri L, Santi C, Tomassini C, Marini F, Bagnoli L, Temperini A (2002) Preparation of a new chiral non-racemic sulfur-containing diselenide and applications in asymmetric synthesis. Chem Eur J 8:1118–1124. doi:10.1002/1521-3765(20020301)8:5<1118:AID-CHEM1118>3.0.CO;2-2
Browne DM, Niyomura O, Wirth T (2007) Catalytic use of selenium electrophiles in cyclizations. Org Lett 9:3169–3171. doi:10.1021/ol071223y
Shahzad SA, Wirth T (2009) Fast synthesis of benzofluorenes by selenium-mediated carbocyclizations. Angew Chem Int Ed 48:2588–2591. doi:10.1002/anie.200806148
Freudendahl DM, Santoro S, Shahzad SA, Santi C, Wirth T (2009) Green chemistry with selenium reagents: development of efficient catalytic reactions. Angew Chem Int Ed 48:8409–8411. doi:10.1002/anie.200903893
Shahzad SA, Venin C, Wirth T (2010) Diselenide- and disulfide-mediated synthesis of isocoumarins. Eur J Org Chem. doi:10.1002/ejoc.201000308
Shahzad SA, Vivant C, Wirth T (2010) Selenium-mediated synthesis of biaryls through rearrangement. Org Lett 12:1364–1367. doi:10.1021/ol100274e
Singh FV, Wirth T (2011) Selenium-catalyzed regioselective cyclization of unsaturated carboxylic acids using hypervalent iodine oxidants. Org Lett 13:6504–6507. doi:10.1021/ol202800k
Zhao L, Li Z, Wirth T (2011) Asymmetric methoxyselenenylations with chiral selenium electrophiles. Eur J Org Chem. doi:10.1002/ejoc.201101373
Santi C, Lorenzo RD, Tidei C, Bagnoli L, Wirth T (2012) Stereoselective selenium catalyzed dihydroxylation and hydroxymethoxylation of alkenes. Tetrahedron 68:10530–10535. doi:10.1016/j.tet.2012.08.078
Engman L (1987) Phenylseleniumtrichloride in organic synthesis. reaction with unsaturated compounds preparation of vinylic chlorides via selenoxide elimination. J Org Chem 52:4086–4094. doi:10.1021/jo00227a026
Engman L (1988) Methods for the introduction of a phenylselenium dichloride group into the alpha-position of carbonyl compounds syntheses of enones. J Org Chem 53:4031–4037. doi:10.1021/jo00252a028
Engman L (1989) Acetoxyselenylation of olefins for the preparation of vinylic and allylic acetates. J Org Chem 54:884–890. doi:10.1021/jo00265a031
Engman L (1991) Organoselenium- and proton-mediated cyclization reactions of allylic amides and thioamides syntheses of 2-oxazolines and 2-thiazolines. J Org Chem 56:3425–3430. doi:10.1021/jo00010a045
Engman L (1993) Functional group manipulation via organoselenium- and halogen-induced cyclofunctionalization/hydrolysis of allylic benzimidates, tertiary benzamides, and benzamidines regioflexible synthesis of amino alcohol derivatives. J Org Chem 58:2394–2401. doi:10.1021/jo00061a009
Besev M, Engman L (2000) Pyrrolidines from β-aminoselenides via radical cyclization diastereoselectivity control by the N-substituent. Org Lett 2:1589–1592. doi:10.1021/ol005829x
Vasil’ev A, Engman L (2000) Novel preparation of α, β-unsaturated aldehydes benzeneselenolate promotes elimination of HBr from α-bromoacetals. J Org Chem 65:2151–2162. doi:10.1021/jo9917644
Ericsson C, Engman L (2001) Diastereocontrol by trialkylaluminums in the synthesis of tetrahydrofurans via radical cyclization. Org Lett 3:3459–3462. doi:10.1021/ol016429s
Besev M, Engman L (2002) Diastereocontrol by a hydroxyl auxiliary in the synthesis of pyrrolidines via radical cyclization. Org Lett 4:3023–3025. doi:10.1021/ol026038t
Berlin S, Ericsson C, Engman L (2002) Construction of tetrahydrofuran-3-ones from readily available organochalcogen precursors via radical carbonylation/reductive cyclization. Org Lett 4:3–6. doi:10.1021/ol016127q
Back TG, Muralidharan KR (1991) Formation and electrophilic reactions of benzeneselenenyl p-toluenesulfonate preparation and properties of addition products with acetylenes. J Org Chem 56:2781–2787. doi:10.1021/jo00008a039
Back TG, Krishna MV (1991) Synthesis of castasterone and formal synthesis of brassinolide from stigmasterol via a selenosulfonation approach. J Org Chem 56:454–457. doi:10.1021/jo00001a089
Back TG, Daniel W (1995) Diels-Alder reactions of 1-(phenylseleno)-2-(p-toluenesulfonyl)ethyne—a novel dienophile and ketene equivalent. Synlett 11:1123–1124. doi:10.1055/s-1995-5216
Back TG, Dyck BP, Parvez M (1995) 1,3-Diselenetanes and 1,3-dithietanes derived from camphor formation, structure, stereochemistry, and oxidation to selenoxide and sulfoxide products. J Org Chem 60:703–710. doi:10.1021/jo00108a038
Back TG, Dyck BP (1996) Asymmetric cyclization of unsaturated alcohols and carboxylic acids with camphor-based selenium electrophiles. Chem Commun 22:2567–2568. doi:10.1039/CC9960002567
Back TG, Siqiao N (1998) Asymmetric methoxyselenenylations with camphor-based selenium electrophiles. J Chem Soc Perkin Trans 1:3123–3124. doi:10.1039/A806707D
Back TG, Bethell RJ, Parvez M, Wehrli D (1998) Additions of organocopper reagents and heteroatom nucleophiles to 1-phenylseleno-2-(p-toluenesulfonyl)ethyne preparation of vinyl and allenic sulfones and formation of michael, anti-michael, and rearrangement products. J Org Chem 63:7908–7919. doi:10.1021/jo981224r
Back TG, Bethell RJ, Parvez M, Taylor JA, Wehrli D (1999) Cycloaddition reactions of 1-phenylseleno-2-(p-toluenesulfonyl)ethyne. J Org Chem 64:7426–7432. doi:10.1021/jo990730t
Back TG, Dyck BP, Nan S (1999) Asymmetric electrophilic methoxyselenenylations and cyclizations with 3-camphorseleno derivatives. Tetrahedron 55:3191–3208. doi:10.1016/S0040-4020(98)01133-8
Back TG, Moussa Z (2000) New chiral auxiliaries for highly stereoselective asymmetric methoxyselenenylations. Org Lett 2:3007–3009. doi:10.1021/ol000187z
Back TG, Moussa Z, Parvez M (2002) Asymmetric methoxyselenenylations and cyclizations with 3-camphorseleno electrophiles containing oxime substituents at C-2 formation of an unusual oxaselenazolefrom an oxime-substituted selenenyl bromide. J Org Chem 67:499–509. doi:10.1021/jo016061c
Back TG, Moussa Z, Parvez M (2004) Preparation and X-ray crystal structures of two aliphatic selenenyl bromides stabilized by N–Se coordination. Phosphorus, Sulfur, Silicon 179:2569–2579
Mercier EA, Smith CD, Parvez M, Back TG (2012) Cyclic seleninate esters as catalysts for the oxidation of sulfides to sulfoxides, epoxidation of alkenes, and conversion of enamines to α-hydroxyketones. J Org Chem 77:3508–3517. doi:10.1021/jo300313v
Detty MR, Zhou F, Friedman AE (1996) Positive halogens from halides and hydrogen peroxide with organotellurium catalysts. J Am Chem Soc 118:313–318. doi:10.1021/ja953187g
Francavilla C, Bright FV, Detty MR (1999) Dendrimeric catalysts for the activation of hydrogen peroxide. Increasing activity per catalytic phenylseleno group in successive generations. Org Lett 1:1043–1046. doi:10.1021/ol990836a
Francavilla C, Drake MD, Bright FV, Detty MR (2001) Dendrimericorganochalcogen catalysts for the activation of hydrogen peroxide: improved catalytic activity through statistical effects and cooperativity in successive generations. J Am Chem Soc 123:57–67. doi:10.1021/ja002649+
Takada H, Metzner P, Philouze C (2001) First chiral selenium ylides used for asymmetric conversion of aldehydes into epoxides. Chem Commun. doi:10.1039/B106063P
Drake MD, Bateman MA, Detty MR (2003) Substituent effects in arylseleninic acid-catalyzed bromination of organic substrates with sodium bromide and hydrogen peroxide. Organometallics 22:4158–4162. doi:10.1021/om0340239
Drake MD, Bright FV, Detty MR (2003) Dendrimeric organochalcogen catalysts for the activation of hydrogen peroxide: origins of the “dendrimer effect” with catalysts terminating in phenylseleno groups. J Am Chem Soc 125:12558–12566. doi:10.1021/ja0367593
Goodman MA, Detty MR (2004) Selenoxides as catalysts for the activation of hydrogen peroxide bromination of organic substrates with sodium bromide and hydrogen peroxide. Organometallics 23:3016–3020. doi:10.1021/om049908e
Braddock DC, Cansell G, Hermitage SA (2004) (Diacetoxyiodo)benzene-lithium bromide as a convenient electrophilic Br+ source. Synlett. doi:10.1055/s-2004-815410
Denmark SE, Edwards MG (2006) On the mechanism of the selenolactonization reaction with selenenyl halides. J Org Chem 71:7293–7306. doi:10.1021/jo0610457
Denmark SE, Collins WR (2007) Lewis base activation of lewis acids: development of a lewis base catalyzed selenolactonization. Org Lett 9:3801–3804. doi:10.1021/ol701617d
Denmark SE, Beutner GL (2008) Lewis base catalysis in organic synthesis. Angew Chem Int Ed 47:1560–1638. doi:10.1002/anie.200604943
Bennett SM, Tang Y, McMaster D, Bright FV, Detty MR (2008) A xerogel-sequestered selenoxide catalyst for brominations with hydrogen peroxide and sodium bromide in an aqueous environment. J Org Chem 73:6849–6852. doi:10.1021/jo801234e
Denmark SE, Collins WR, Cullen MD (2009) Observation of direct sulfenium and selenenium group transfer from thiiranium and seleniranium ions to alkenes. J Am Chem Soc 131:3490–3492. doi:10.1021/ja900187y
Denmark SE, Kalyani D, Collins WR (2010) Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions. J Am Chem Soc 132:15752–15765. doi:10.1021/ja106837b
Mellegaard SR, Tunge JA (2004) Selenium-catalyzed halolactonization: nucleophilic activation of electrophilic halogenating reagents. J Org Chem 69:8979–8981. doi:10.1021/jo048460o
Wang C, Tunge JA (2004) Selenocatalytic α-halogenation. Chem Commun. doi:10.1039/B410576A
Tunge JA, Mellegaard SR (2004) Selective selenocatalytic allylic chlorination. Org Lett 6:1205–1207. doi:10.1021/ol036525o
Mellegaard SR, Wang C, Tunge JA (2006) Selenium-catalyzed oxidative halogenation. Tetrahedron 62:7191–7198. doi:10.1016/j.tet.2005.12.072
Tay DW, Tsoi IT, Er JC, Leung GYC, Yeung Y-Y (2013) Lewis basic selenium catalyzed chloroamidation of olefins using nitriles as the nucleophiles. Org Lett 15:1310–1313. doi:10.1021/ol400249x
Chen F, Tan CK, Yeung Y-Y (2013) C2-symmetric cyclic selenium-catalyzed enantioselective bromoaminocyclization. J Am Chem Soc 135:1232–1235. doi:10.1021/ja311202e
Yokomatsu T, Iwasawa H, Shibuya S (1992) Enantioselective halolactonisation of bis-γ, δ-unsaturated carboxylic acid derivatives: use of a sultam and oxazolidine-2-ones as chiral auxiliary. J Chem Soc Chem Commun. doi:10.1039/C39920000728
Eissen M, Lenoir D (2008) Electrophilic bromination of alkenes: environmental, health and safety aspects of new alternative methods. Chem Eur J 14:9830–9841. doi:10.1002/chem.200800462
Braddock DC, Cansella G, Hermitage SA (2006) Ortho-substituted iodobenzenes as novel organocatalysts for bromination of alkenes. Chem Commun. doi:10.1039/B604130B
Braddock DC, Cansell G, Hermitage SA, White AJP (2006) Bromoiodinanes with an I(III)–Br bond: preparation, X-ray crystallography and reactivity as electrophilic brominating agents. Chem Commun. doi:10.1039/B600455E
Ahmad SM, Braddock DC, Cansell G, Hermitage SA, Redmond JM, White AJP (2007) Amidines as potent nucleophilic organocatalysts for the transfer of electrophilic bromine from N-bromosuccinimide to alkenes. Tetrahedron Lett 48:5948–5952. doi:10.1016/j.tetlet.2007.06.112
Braddock DC, Hermitage SA, Kwok L, Pouwer R, Redmond JM, White AJP (2009) The generation and trapping of enantiopure bromonium ions. Chem Commun. doi:10.1039/B816914D
Braddock DC, Marklew JS, Thomas AJF (2011) Enantiospecific bromonium ion generation and intramolecular capture: a model system for asymmetric bromonium ion-induced polyene cyclisations. Chem Commun 47:9051–9053. doi:10.1039/C1CC13619D
Bonney KJ, Braddock DC (2012) A unifying stereochemical analysis for the formation of halogenated C15-acetogenin medium-ring ethers from laurencia species via intramolecular bromonium ion assisted epoxide ring-opening and experimental corroboration with a model epoxide. J Org Chem 77:9574–9584. doi:10.1021/jo301580c
Tan CK, Zhou L, Yeung Y-Y (2011) Organocatalytic enantioselective halolactonizations: strategies of halogen activation. Synlett. doi:10.1055/s-0030-1260578
Monaco MR, Bella M (2011) A formidable challenge: catalytic asymmetric dichlorination. Angew Chem Int Ed 50:11044–11046. doi:10.1002/anie.201104843
Denmark SE, Kuester WE, Burk MT (2012) Catalytic, asymmetric halofunctionalization of alkenes—a critical perspective. Angew Chem Int Ed 51:10938–10953. doi:10.1002/anie.201204347
Snyder SA, Treitler DS, Brucks AP (2011) Halonium-induced cyclization reactions. Aldrichimica Acta 44:27
Hennecke U (2012) New catalytic approaches towards the enantioselective halogenation of alkenes. Chem Asian J 7:456–465. doi:10.1002/asia.201100856
Zhou L, Tan CK, Jiang X, Chen F, Yeung Y-Y (2010) Asymmetric bromolactonization using amino-thiocarbamate catalyst. J Am Chem Soc 132:15474–15476. doi:10.1021/ja1048972
Tan CK, Zhou L, Yeung Y-Y (2011) Aminothiocarbamate-catalyzed asymmetric bromolactonization of 1,2-disubstituted olefinic acids. Org Lett 13:2738–2741. doi:10.1021/ol200840e
Zhou L, Chen J, Tan CK, Yeung Y-Y (2011) Enantioselective bromoaminocyclization using amino–thiocarbamate catalysts. J Am Chem Soc 133:9164–9167. doi:10.1021/ja201627h
Jiang X, Tan CK, Zhou L, Yeung Y-Y (2012) Enantioselective bromolactonization using an S-alkyl thiocarbamate catalyst. Angew Chem Int Ed 51:7771. doi:10.1002/anie.201202079
Tan CK, Le C, Yeung Y-Y (2012) Enantioselective bromolactonization of cis-1,2-disubstituted olefinic acids using an amino-thiocarbamate catalyst. Chem Commun 48:5793–5795. doi:10.1039/C2CC31148H
Chen J, Zhou L, Yeung Y-Y (2012) A highly enantioselective approach towards 2-substituted 3-bromopyrrolidines. Org Biomol Chem 10:3808–3811. doi:10.1039/C2OB25327E
Chen J, Zhou L, Tan CK, Yeung Y-Y (2012) An enantioselective approach toward 3,4-dihydroisocoumarin through the bromocyclization of styrene-type carboxylic acids. J Org Chem 77:999–1009. doi:10.1021/jo202221c
Yeung Y-Y, Corey EJ (2007) An efficient process for the bromolactamization of unsaturated acids. Tetrahedron Lett 48:7567–7570. doi:10.1016/j.tetlet.2007.08.113
Chen F, Jiang X, Er JC, Yeung Y-Y (2010) Molecular sieves as an efficient and recyclable catalyst for bromolactonization and bromoacetoxylation reactions. Tetrahedron Lett 51:3433–3435. doi:10.1016/j.tetlet.2010.04.113
Zhou L, Tan CK, Zhou J, Yeung Y-Y (2010) Facile, efficient, and catalyst-free electrophilic aminoalkoxylation of olefins: scope and application. J Am Chem Soc 132:10245–10247. doi:10.1021/ja104168q
Zhou L, Chen J, Zhou J, Yeung Y-Y (2011) N-bromosuccinimide promoted one-pot synthesis of guanidine: scope and mechanism. Org Lett 13:5804–5807. doi:10.1021/ol202402y
Chen J, Cheng S, Zhou L, Yeung Y-Y (2011) Scope and mechanistic studies of electrophilic alkoxyetherification. Org Lett 13:6456–6459. doi:10.1021/ol202751s
Tan CK, Chen F, Yeung Y-Y (2011) Studies toward lewis basic thiocarbamate and thiourea mediated bromolactonization: the effect of a trace amount of water on the reactivity and enantioselectivity. Tetrahedron Lett 52:4892–4895. doi:10.1016/j.tetlet.2011.07.049
Zhou L, Zhou J, Tan CK, Chen J, Yeung Y-Y (2011) N-bromosuccinimide initiated one-pot synthesis of imidazoline. Org Lett 13:2448–2451. doi:10.1021/ol2006902
Zhou J, Zhou L, Yeung Y-Y (2012) Multicomponent approach in the synthesis of 2,2,6-trisubstituted morpholine derivatives. Org Lett 14:5250–5253. doi:10.1021/ol3024105
Cheng YA, Chen T, Tan CK, Heng JJ, Yeung Y-Y (2012) Efficient medium ring size bromolactonization using a sulfur-based zwitterionic organocatalyst. J Am Chem Soc 134:16492–16495. doi:10.1021/ja307210n
Cheng YA, Yu WZ, Yeung Y-Y (2014) Recent advances in asymmetric intra- and intermolecular halofunctionalizations of alkenes. Org Biomol Chem 12:2333–2343. doi:10.1039/C3OB42335B
Tan CK, Er JC, Yeung Y-Y (2014) Synthesis of chiral butenolides using amino-thiocarbamate-catalyzed asymmetric bromolactonization. Tetrahedron Lett 55:1243–1246. doi:10.1016/j.tetlet.2014.01.009
Haas J, Piguel S, Wirth T (2002) Reagent-controlled stereoselective iodolactonizations. Org Lett 4:297–300. doi:10.1021/ol0171113
Kang SH, Lee SB, Park CM (2003) Catalytic enantioselective iodocyclization of γ-hydroxy-cis-alkenes. J Am Chem Soc 125:15748–15749. doi:10.1021/ja0369921
Wang M, Gao LX, Mai WP, Xia AX, Wang F, Zhang SB (2004) Enantioselective iodolactonization catalyzed by chiral quaternary ammonium salts derived from cinchonidine. J Org Chem 69:2874–2876. doi:10.1021/jo035719e
Sakakura A, Ukai A, Ishihara K (2007) Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites. Nature 445:900–903. doi:10.1038/nature05553
Ning Z, Jin R, Ding J, Gao L (2009) Enantioselective iodolactonizations of 4-pentenoic acid derivatives mediated by chiral salen-Co(II) complex. Synlett. doi:10.1055/s-0029-1217806
Chen G, Ma S (2010) Enantioselective halocyclization reactions for the synthesis of chiral cyclic compounds. Angew Chem Int Ed 49:8306–8308. doi:10.1002/anie.201003114
Murai K, Matsushita T, Nakamura A, Fukushima S, Shimura M, Fujioka H (2010) Asymmetric bromolactonization catalyzed by a C3-symmetric chiral trisimidazoline. Angew Chem Int Ed 49:9174–9177. doi:10.1002/anie.201005409
Veitch GE, Jacobsen EN (2010) Tertiary aminourea-catalyzed enantioselective iodolactonization. Angew Chem Int Ed 49:7332–7335. doi:10.1002/anie.201003681
Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W (2010) Enantioselective bromolactonization of conjugated (Z)-enynes. J Am Chem Soc 132:3664–3665. doi:10.1021/ja100173w
Whitehead DC, Yousefi R, Jaganathan A, Borhan B (2010) An organocatalytic asymmetric chlorolactonization. J Am Chem Soc 132:3298–3300. doi:10.1021/ja100502f
Denmark SE, Burk MT, Hoover AJ (2010) On the absolute configurational stability of bromonium and chloronium ions. J Am Chem Soc 132:1232–1233. doi:10.1021/ja909965h
Denmark SE, Burk MT (2010) Lewis base catalysis of bromo- and iodolactonization, and cycloetherification. Proc Natl Acad Sci USA 107:20655–20660. doi:10.1073/pnas.1005296107
Huang S-X, Ding K (2011) Asymmetric bromoamination of chalcones with a privileged N, N′-dioxide/scandium(III) catalyst. Angew Chem Int Ed 50:7734–7736. doi:10.1002/anie.201101076
Hennecke U, Mΰller CH, Fröhlich R (2011) Enantioselective haloetherification by asymmetric opening of meso-halonium ions. Org Lett 13:860–863. doi:10.1021/ol1028805
Fang C, Paull DH, Hethcox JC, Shugrue CR, Martin SF (2012) Enantioselective iodolactonization of disubstituted olefinic acids using a bifunctional catalyst. Org Lett 14:6290–6293. doi:10.1021/ol3030555
Tungen JE, Nolsøe JMJ, Hansen TV (2012) Asymmetric iodolactonization utilizing chiral squaramides. Org Lett 14:5884–5887. doi:10.1021/ol302798g
Paull DH, Fang C, Donald JR, Pansick AD, Martin SF (2012) Bifunctional catalyst promotes highly enantioselective bromolactonizations to generate stereogenic C-Br bonds. J Am Chem Soc 134:11128–11131. doi:10.1021/ja305117m
Ikeuchi K, Ido S, Yoshimura S, Asakawa T, Inai M, Hamashima Y, Kan T (2012) Catalytic desymmetrization of cyclohexadienes by asymmetric bromolactonization. Org Lett 14:6016–6019. doi:10.1021/ol302908a
Lee HJ, Kim DY (2012) Catalytic enantioselective bromolactonization of alkenoic acids in the presence of palladium complexes. Tetrahedron Lett 5:6984–6986. doi:10.1016/j.tetlet.2012.10.051
Wang Y-M, Wu J, Hoong C, Rauniyar V, Toste FD (2012) Enantioselective halocyclization using reagents tailored for chiral anion phase-transfer catalysis. J Am Chem Soc 134:12928–12931. doi:10.1021/ja305795x
Denmark SE, Burk MT (2012) Enantioselective bromocycloetherification by lewis base/chiral brønsted acid cooperative catalysis. Org Lett 14:256–259. doi:10.1021/ol203033k
Fang C, Paull DH, Hethcox JC, Shugrue CR, Martin SF (2013) Enantioselective iodolactonization of disubstituted olefinic acids using a bifunctional catalyst. Org Lett 15:972. doi:10.1021/ol400125b
Grossman RB, Trupp RJ (1998) The first reagent-controlled asymmetric halolactonizations dihydroquinidine-halogen complexes as chiral sources of positive halogen ion. Can J Chem 76:1233–1237. doi:10.1139/v98-153
Balkrishna SJ, Bhakuni BS, Chopra D, Kumar S (2010) Cu-catalyzed efficient synthetic methodology for ebselen and related Se–N heterocycles. Org Lett 12:5394–5397. doi:10.1021/ol102027j
Balkrishna SJ, Bhakuni BS, Kumar S (2011) Copper catalyzed/mediated synthetic methodology for ebselen and related isoselenazolones. Tetrahedron 67:9565–9575. doi:10.1016/j.tet.2011.09.141
Bhakuni BS, Balkrishna SJ, Kumar A, Kumar S (2012) An efficient copper mediated synthetic methodology for benzo[d]isothiazol-3(2H)-ones and related sulfur–nitrogen heterocycles. Tetrahedron Lett 53:1354–1357. doi:10.1016/j.tetlet.2012.01.003
Balkrishna SJ, Prasad CD, Panini P, Detty MR, Chopra D, Kumar S (2012) Isoselenazolones as catalysts for the activation of bromine: bromolactonization of alkenoic acids and oxidation of alcohols. J Org Chem 77:9541–9552. doi:10.1021/jo301486c
Prasad CD, Balkrishna SJ, Kumar A, Bhakuni BS, Shrimali K, Biswas S, Kumar S (2013) Transition-metal-free synthesis of unsymmetrical diaryl chalcogenides from arenes and diaryl dichalcogenides. J Org Chem 78:1434–1443. doi:10.1021/jo302480j
Balkrishna SJ, Kumar S, Azad GK, Bhakuni BS, Panini P, Ahalawat N, Tomar RS, Kumar S (2014) An ebselen like catalyst with enhanced GPx activity via a selenol intermediate. Org Biomol Chem 12:1215–1219. doi:10.1039/C4OB00027G
Balkrishna SJ, Hodage A, Kumar S, Panini P, Kumar S (2014) Sensitive and regenerableorganochalcogen probes for the colorimetric detection of thiols. RSC Adv 4:11535–11538. doi:10.1039/C4RA00381
Verma A, Jana S, Prasad CD, Yadav A, Kumar S (2016) Organoselenium and DMAP co-catalysis: regioselective synthesis of medium-sized halolactones and bromooxepanes from unactivated alkenes. Chem Commun 52:4179–4182. doi:10.1039/C5CC10245F
Brunner H, Bugler J, Nuber B (1995) Enantioselective catalysis 98 preparation of 9-amino(9-deoxy)cinchona alkaloids. Tetrahedron Asymmetry 6:1699–1702. doi:10.1016/0957-4166(95)00215-B
Cassani C, Rapún R-M, Arceo E, Bravo F, Melchiorre P (2013) Synthesis of 9-amino(9-deoxy)epi cinchona alkaloids, general chiral organocatalysts for the stereoselective functionalization of carbonyl compounds. Nat Protoc 8:325–344. doi:10.1038/nprot.2012.155
Wang W, Ma X, Wan J, Cao J, Tang Q (2012) Preparation and confinement effect of a heterogeneous 9-amino-9-deoxy-epi-cinchonidine organocatalyst for asymmetric aldol addition in aqueous medium. Dalton Trans 41:5715–5726. doi:10.1039/C2DT12390H
Singh VP, Singh HB, Butcher RJ (2011) Photoluminescentselenospirocyclic and selenotetracyclic derivatives by domino reactions of amines and imines. Chem Commun 47:7221–7223. doi:10.1039/C1CC12152A
Selvakumar K, Shah P, Singh HB, Butcher RJ (2011) Synthesis, structure, and glutathione peroxidase-like activity of amino acid containing ebselen analogues and diaryl diselenides. Chem Eur J 17:12741–12755. doi:10.1002/chem.201100930
Zade SS, Panda S, Tripathi SK, Singh HB, Wolmershäuser G (2004) Convenient synthesis, characterization and GPx-like catalytic activity of novel ebselen derivatives. Eur J Org Chem. doi:10.1002/ejoc.200400326
Selvakumar K, Singh HB, Butcher RJ (2011) Strained dimethyl 2-(bromoselanyl)-5-tert-butylisophthalate: a reactive precursor for the synthesis of ebselen analogs. Tetrahedron Lett 52:6831–6834. doi:10.1016/j.tetlet.2011.10.067
Bhabak KP, Mugesh G (2007) Synthesis, characterization, and antioxidant activity of some ebselen analogues. Chem Eur J 13:4594–4601. doi:10.1002/chem.200601584
Sarma BK, Mugesh G (2008) Antioxidant activity of the anti-inflammatory compound ebselen: a reversible cyclization pathway via selenenic and seleninic acid intermediates. Chem Eur J 14:10603–10614. doi:10.1002/chem.200801258
Bhabak KP, Mugesh G (2009) Amide-based glutathione peroxidase mimics: effect of secondary and tertiary amide substituents on antioxidant activity. Chem Asian J 4:974–983. doi:10.1002/asia.200800483
Sarma BK, Manna D, Minoura M, Mugesh G (2010) Synthesis, structure, spirocyclization mechanism, and glutathione peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J Am Chem Soc 132:5364–5374. doi:10.1021/ja908080u
Acknowledgments
The authors are thankful to Department of Science and Technology (DST), New Delhi, India, Department of Atomic Energy (DAE-BRNS) Mumbai, and IISER Bhopal for financial support. S.K. is grateful to Dr. Deepak Chopra (IISER Bhopal) for solving the crystal structures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balkrishna, S.J., Kumar, S., Kumar, A. et al. Cinchona-Alkaloids Based Isoselenazolones: Synthesis and Their Catalytic Reactivity in Asymmetric Bromolactonization of Alkenoic Acid. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 86, 589–600 (2016). https://doi.org/10.1007/s40010-016-0306-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-016-0306-9