Abstract
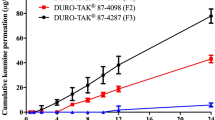
The purpose of this study was to investigate the feasibility of developing transdermal drug delivery system for ketotifen used for asthma, allergic conjunctivitis, and rhinitis. The permeation of ketotifen in silicone, polyisobutylene, styrene–butadiene–styrene and acrylic-rubber hybrid and acrylic pressure sensitive adhesive matrix was evaluated. Due to good adhesion force and high permeability, acrylic-rubber hybrid adhesive was chosen. Permeation rate was found to increase linearly as the drug concentration in acrylic-rubber hybrid adhesive increased. However, when the drug concentration was 5 % or more in the matrix, recrystallization of ketotifen was observed. The recrystallization process was not inhibited by crystallization inhibitors tested. Significant increase in flux was obtained using Brij® 30, Crovol® A40, Span® 80 and Lauroglycol® FCC as permeation enhancers.



Similar content being viewed by others
References
Ahn TS, Lee JP, Kim J, Oh SY, Chun MK, Choi HK (2013) Effect of pressure sensitive adhesive and vehicles on permeation of terbinafine across porcine hoof membrane. Arch Pharm Res 36:1403–1409
Allen LV, Popovich NG, Ansel HC (2004) Ansel’s pharmaceutical dosage forms and drug delivery system, 9th edn. Lippincott Williams & Wilkins, Philadelphia
Aungst BJ (2012) Absorption enhancers: application and advances. AAPS J 14:10–18
Berba J, Goranson S, Langle J, Banakar UV (1991) In vitro release of selected nonsteroidal anti-inflammatory analgesics from reservoir type transdermal formulations. Drug Dev Ind Pharm 17:55–65
Brown MB, Martin GP, Jones SA, Akomeah FK (2006) Demal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–187
Cho YJ, Choi HK (1998) Enhancement of percutaneous absorption of ketoprofen: effect of vehicles and adhesive matrix. Int J Pharm 169:95–104
Choi YK, Kim JJ, Lee JS, Kim SS (2011) Transdermal patch for delivery of fentanyl. KR Patent Application No. KR20110022958
Grant SM, Goa KL, Fitton A, Sorkin EM (1990) Ketotifen. A review of its phamacodynamic and phamacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs 40:412–448
Joly F, Bessou G, Beveniste J, Ninio E (1987) Ketotifen inhibits paf-acether biosynthesis and beta-hexosaminidase release in mouse mast cells stimulated with antigen. Eur J Pharmacol 144:133–139
Kim JH, Choi HK (2002) Effect of additives on the crystallization and the permeation of ketoprofen from adhesive matrix. Int J Pharm 236:81–85
Kim JH, Cho YJ, Choi HK (2000) Effect of vehicles and pressure sensitive adhesive on the permeation of tacrine across hairless mouse skin. Int J Pharm 196:105–113
Kotiyan PN, Vavia PR (2001) Eudragits: role as crystallization inhibitors in drug-in adhesive transdermal systems of estradiol. Eur J Pharm Biopharm 52:173–180
Lee SY, Jeong NY, Oh SY (2013) Modulation of electroosmosis and flux through skin: effect of propylene glycol. Arch Pharm Res. doi:10.1007/s1227201302566
Mahdy AM, Webster NR (2008) Histamine and antihistamines. Anaesth Intensive Care Med 9:324–328
Mallya P, Smith CC (1997) Acrylic saturated rubber hybrid pressure-sensitive adhesives. US Patent No. 5,625,005
Mitragotri S (2000) Synergistic effect of enhancers for transdermal drug delivery. Pham Res 17:1354–1359
Mitragotri S (2004) Breaking the skin barrier. Adv Drug Deliv Rev 56:555–556
Murphy M, Carmichael AJ (2000) Transdermal drug delivery system and skin sensitivity reactions. Incidence and management. Am J Clin Dermatol 1:361–368
Nnane IP, Damani LA, Hutt AJ (1998) Development and validation of stability indicating high-performance liquid chromatographic assay for ketotifen in aqueous and silicon oil formulations. Chromatographia 48:797–802
Peterson TA, Wick SM, Ko C (1997) Design, development, manufacturing, and testing of transdermal drug delivery systems. In: Ghosh TK, Pfister WR, Yum SI (eds) Transdermal and topical drug delivery systems. Interpharm Press, Buffalo, pp 249–297
Rhee YS, Nguyen T, Park ES, Chi SC (2013) Formulation and biopharmaceutical evaluation of a transdermal patch containing aceclofenac. Arch Pharm Res 36:602–607
Schmidt-Redemann B, Brenneisen P, Schmidt-Redemann W, Gonda S (1986) The determination of pharmacokinetic parameters of ketotifen in steady state in young children. Int J Clin Pharmacol Ther Toxicol 24:496–498
Subedi RK, Oh SY, Chun MK, Choi HK (2010) Recent advances in transdermal drug delivery. Arch Pharm Res 33:339–351
Tan HS, Pfister WR (1999) Pressure-sensitive adhesive for transdermal drug delivery systems. Pharm Sci Technol Today 2:60–69
Yagi N, Taniuchi Y, Hamada K, Sudo J, Sekikawa H (2002) Pharmacokinetics of ketotifen fumarate after intravenous, intranasal, oral and rectal administration in rabbits. Biol Pham Bull 25:1614–1618
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (E.-Y. Lee, M.-K. Chun, J.-S. Chang, and H.-K. Choi) declare that they have no conflict of interest. This work was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs (A092018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, EY., Chun, MK., Chang, JS. et al. Development of matrix based transdermal delivery system for ketotifen. Journal of Pharmaceutical Investigation 44, 291–296 (2014). https://doi.org/10.1007/s40005-014-0126-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0126-3