Abstract
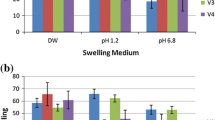
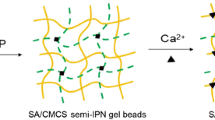
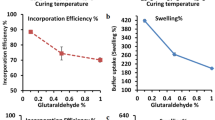
The work investigates the development and optimization of chitosan-alginate beads containing Losartan potassium (LP) through ionotropic/external gelation technique using 32 factorial design. The effect of polymer-blend concentrations i.e. chitosan and sodium alginate on the drug encapsulation efficiency (DEE%), and cumulative drug release after 20 h (R20h %) was optimized. The DEE% of all these beads was within the range of 67.12 ± 1.97–89.81 ± 1.52 % with sustained in vitro drug release of 80.98–97.13 % over 20 h. The in vitro drug release from these beads was followed first order kinetic model. The beads were also characterized by FE-SEM, FTIR and XRD analysis. The swelling of chitosan–alginate beads containing LP were influenced by the pH of the test medium. Chitosan coated alginate beads were developed as oral sustained delivery carriers for LP in order to improve patient compliance, to reduce side effects associated with it and also to reduce the dose/dosing frequency in the management of hypertension.








Similar content being viewed by others
References
Anil KA, Willem FS (2005) Chitosan–alginate multilayer beads for controlled release of ampicillin. Int J Pharm 290:45–54
Fwu LM, Shin SS, Hsing WS (2002) Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr Polym 48:61–72
Gacesa P (1988) Alginate. Carbohydr Polym 8:161–182
GåserØd O, SmidsrØd O, Skjåk BG (1998) Microcapsules of alginate–chitosan. I. A quantitative study of the interaction between alginate and chitosan. Biomaterials 19:1815–1825
GåserØd O, SmidsrØd O, Skjåk BG (1999) Microcapsules of alginate–chitosan. II. A study of capsule stability and permeability. Biomaterials 20:773–783
Gombotz WR, Wee SF (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31:267–285
Illum L (1998) Chitosan and its use as a pharmaceutical excipient. Pharm Res 15:1326–1331
Lee OS, Ha BJ, Park SN, Lee YSN (1997) Studies on the pH-dependent swelling properties and morphologies of chitosan/calcium-alginate complexed beads. Macromol Chem Phys 198:2971–2976
Malakar J, Nayak AK, Pal D (2012) Development of cloxacillin loaded multiple-unit alginate-based floating system by emulsion–gelation method. Int J Biol Macromol 50:138–147
Marc H (2008) Angiotensin-converting enzyme inhibitors, antagonists and calcium blockers. In: Thomas LL, David AW, Victoria FR, William SZ (eds) Foye’s principle of medicinal chemistry. Wolters Kluwer (India) Pvt Ltd, New Delhi, pp 754–755
Murata Y, Maeda T, Miyamoto E, Kawashima S (1993) Preparation of chitosan-reinforced alginate beads-effects of chitosan on gel matrix erosion. Int J Pharm 96:139–145
Naik JB, Mokale VJ (2012) Formulation and evaluation of repaglinide nanoparticles as a sustained release carriers. Novel Sci Int J Pharm Sci 1(5):259–266
Nayak AK, Pal D (2011) Develpment of pH-sensitive tamarind seed polysaccharide alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int J Biol Macromol 49:784–793
NIST/SEMATECH (2013) e Handbook of statistical methods. http://www.itl.nist.gov/div898/handbook/. Assessed 5 Feb 2013.
Pasparakis G, Bouropoulos N (2006) Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int J Pharm 323:34–42
Patil JS, Kamalapur MV, Marapur SC, Kadam DV (2010) Ionotropic gelation and polyelectrolyte complexation: the novel techniques to design hydrogel particulate sustained, modulated drug delivery system: a review. Digest J Nanomater Biostruct 5:241–248
Remuñan LC, Portero A, Lemos M, Vila JJL, Nuñez MJ, Riveiro P, Lopez JM, Piso M, Alonso MJ (2000) Chitosan microspheres for the specific delivery of amoxicillin to the gastric cavity. S T P Pharm Sci 10:69–76
Sezer AD, Akbˆuga J (1995) Controlled release of piroxicam from chitosan beads. Int J Pharm 121:113–116
Shruti C, Gayathri VP, Sanjay KM (2007) Release modulating hydrophilic matrix systems of losartan potassium: optimization of formulation using statistical experimental design. Eur J Pharm Biopharm 66:73–82
Singh B, Sharma V, Chauhan D (2010) Gastro retentive floating sterculia–alginate beads for use in antiulcer drug delivery. Chem Eng Res Des 88:997–1012
SmidsrØd O, Skjåk BG (1990) Alginate as immobilization matrix for cells. Trends Biotechnol 8:71–78
Sriamornsak P, Kennedy RA (2008) Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int J Pharm 358(1–2):205–213
USP-NF (2007) The official compendia of standards. USP 30/NF 25; 1092-the dissolution procedure: development and validation.
Xiudong L, Weiming X, Qun L, Weiting Y, Yingli F, Xin X, Xiaojun M, Quan Y (2004) Swelling behaviour of alginate–chitosan microcapsules prepared by external gelation or internal gelation technology. Carbohydr Polym 56:459–464
Yongmei X, Changyou Z, Lihong F, Le W, Hua Z (2007) Preparation of dual crosslinked alginate–chitosan blends gel beads and in vitro controlled release in oral site-specific drug delivery system. Int J Pharm 336:329–337
Acknowledgments
The authors are grateful to Defence Research and Development Organisation (DRDO) New Delhi, Govt. of India, for providing financial support, in terms of Extramural Research Project [ERIP/ER/0903820/M/01/1379]. The authors also like to thank Merlin Pharma Pvt. Ltd. Mumbai, Maharashtra, India for providing Losartan potassium free sample of drug and India Sea Foods, Chitin Division, Kannamaly, Kochi-8, India, for providing chitosan as a gift sample.
Conflict of interest
Authors have no conflict of interest regarding this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokale, V., Jitendra, N., Yogesh, S. et al. Chitosan reinforced alginate controlled release beads of losartan potassium: design, formulation and in vitro evaluation. Journal of Pharmaceutical Investigation 44, 243–252 (2014). https://doi.org/10.1007/s40005-014-0122-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0122-7