Abstract
Purpose
Few individuals that are latently infected with M. tuberculosis latent tuberculosis infection(LTBI) progress to active disease. We investigated risk factors for LTBI and active pulmonary tuberculosis (PTB) in Germany.
Methods
Healthy household contacts (HHCs), health care workers (HCWs) exposed to M. tuberculosis and PTB patients were recruited at 18 German centres. Interferon-γ release assay (IGRA) testing was performed. LTBI risk factors were evaluated by comparing IGRA-positive with IGRA-negative contacts. Risk factors for tuberculosis were evaluated by comparing PTB patients with HHCs.
Results
From 2008–2014, 603 HHCs, 295 HCWs and 856 PTBs were recruited. LTBI was found in 34.5% of HHCs and in 38.9% of HCWs. In HCWs, care for coughing patients (p = 0.02) and longstanding nursing occupation (p = 0.04) were associated with LTBI. In HHCs, predictors for LTBI were a diseased partner (odds ratio 4.39), sexual contact to a diseased partner and substance dependency (all p < 0.001). PTB was associated with male sex, low body weight (p < 0.0001), alcoholism (15.0 vs 5.9%; p < 0.0001), glucocorticoid therapy (7.2 vs 2.0%; p = 0.004) and diabetes (7.8 vs. 4.0%; p = 0.04). No contact developed active tuberculosis within 2 years follow-up.
Conclusions
Positive IGRA responses are frequent among exposed HHCs and HCWs in Germany and are poor predictors for the development of active tuberculosis.
Similar content being viewed by others
Introduction
Tuberculosis incidence has declined in Western Europe [1]. In 2014, the notified incidence in the European Union and European Economic Area (EU/EEA) was 14.2 cases per 100,000 population [1], in some countries only 5 cases per 100,000 [1]. Aiming at further reduction, the WHO advocated a target tuberculosis incidence of <1 cases per 1,000,000 in low incidence countries, i.e. the consensual tuberculosis elimination threshold [2].
Tuberculosis prevention relies on early case finding and on the identification of persons latently infected with Mycobacterium tuberculosis (LTBI) [3]. LTBI is defined by a positive response to the tuberculin skin test (TST) or an interferon-release assay (IGRA) without tuberculosis associated symptoms or signs. It is unclear whether the test results reflect viable bacilli in the human host. False positive results can be found, especially in populations of low prevalence.
Approximately 9% of healthy persons in Western Europe have a positive TST or IGRA test result [4]. If the whole population was screened with subsequent preventive chemotherapy for all who tested positive, the number needed to treat to prevent one case would not be cost effective. LTBI screening and treatment is, therefore, only performed in populations with an a priori higher risk for the disease [5], e.g. house hold contacts.
Contact tracing identifies 16–44% of close household contacts of contagious patients with LTBI [6, 7]. Nevertheless, only a small fraction develops active tuberculosis despite the absence of preventive chemotherapy [8]. Predictive markers of progression to active tuberculosis are lacking. The number of latently infected contacts requiring preventive chemotherapy to prevent a single case of tuberculosis is >1:30 in Western Europe [8]. Adherence to recommendations for preventive chemotherapy is poor in Germany [9].
To improve prevention and to target individuals for preventive chemotherapy more precisely, additional knowledge of risk factors for of LTBI and tuberculosis is needed. Therefore, our consortium collected epidemiological and clinical data from pulmonary tuberculosis (PTB) patients and close contacts.
Methods
This observational, multicentre, prospective study was conducted by the German research consortium on “pulmonary tuberculosis—host and pathogen determinants of resistance and disease progression—(TB or not TB)”. It was approved by the ethics committee of the University of Lübeck (reference 07–125) and adopted by the ethics committees of all 18 participating centres.
Household contacts (HHCs) were recruited at three municipal healthcare centres (Frankfurt, Hamburg, Hannover). They were suitable for enrolment if they were asymptomatic with no signs of tuberculosis on chest X-ray, were exposed >8 h to patients with acid-fast bacilli (AFB) in the sputum or >40 h in AFB negative, culture-confirmed PTB. Their last unprotected exposure was ≥8 weeks prior to enrolment.
Healthcare workers (HCWs) with ongoing professional contact to patients with AFB sputum smear-positive tuberculosis, a cumulative exposure of ≥2 years, and no signs or symptoms of tuberculosis were recruited at 18 German respiratory medicine centres.
Patients with current or previous PTB that was culture confirmed from sputum or a broncho-pulmonary specimen were enrolled. Patients were excluded if they were prison inmates, subjects under guardianship or soldiers as this interferes with their informed consent.
Clinical and demographic data were captured on an ad hoc standardised questionnaire.
This questionnaire was amended during the first 3 years resulting in some data on medical and social risk factors. IGRAs were performed on blood from contact persons by the QuantiFERON Gold In-Tube® (QFT; Cellestis Qiagen, Australia) or T-Spot.TB® (Elispot; Oxford Immunotec, Oxford, UK) at the physician’s discretion. TST was not regularly performed since national recommendations regarding the use of the TST were amended during the study [10].
At the start of the study, a two-step approach was recommended involving both, TST and IGRA but TST was abandoned in 2011. The study protocol required an obligatory IGRA and an optional TST.
After 2 years, HHCs were followed up by health authorities; HCWs by a study physician. A questionnaire was sent to the participants. Notified tuberculosis cases would have been reported by the participating health authorities.
Statistical analyses
Variables were described using relative frequencies (percentages) and means (standard deviations—SD) or medians (interquartile ranges—IQR) according to their parametric distribution, respectively.
Chi-squared test and student t test or Mann–Whitney U test were applied appropriately to assess significant differences. Appropriate univariate and multivariate logistic regression analyses were performed.
Two-tailed p values <0.05 were considered significant. Analyses were carried out with STATA®13 software (StataCorp, College Station, TX, USA).
Results
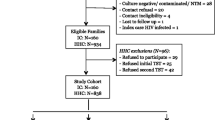
From December 2008 to December 2014 we recruited 1754 individuals, i.e. 603 HHCs, 295 HCWs, and 856 PTB patients (Table 1). 81 HHCs and 15 HCWs were excluded from further analyses due to a missing IGRA result. Among contact persons (Table 2), HCWs were older than HHCs (mean ± SD: 48.0 ± 5.0- vs 40.6 ± 9.3 years; p < 0.0001), were less likely to smoke (23.5 vs 46.4%; p < 0.0001), more likely to be female (88.0 vs 53.6%; p < 0.0001), and to be born in Germany (88.4 vs 53.2%; p < 0.0001) by a German mother (85.9 vs 43.6%; p < 0.0001). An IGRA result was available in 522 HHCs and 280 HCWs, of which 180 (34.5%) and 109 (38.9%) had a positive result, respectively. IGRA results had no gender preference. The proportions of males were 47.8 vs 45.6% (p = 0.64) among HHCs and 21.3 vs 22.5% among HCWs (p = 0.82) with a positive vs. negative IGRA result, respectively. Most of the initial patients had a TST, but due to changed recommendations, later patients did not necessarily undergo skin testing. Policy changes were adapted by the centres with variable delay. For HCWs, neither a well-defined period of exposure nor the moment of last unprotected exposure could be determined. Preventive therapy is not recommended in this setting; therefore, no data about preventive chemotherapy was obtained. Most HHCs were recruited about 6–8 weeks after their last unprotected exposure, i.e. before isoniazid preventive therapy (IPT) was initiated. Therefore, data on IPT was only available in about one-third of the individuals. IPT was administered more often in IGRA-positive persons (11 out of 59 subjects; 18.6%) than IGRA-negative subjects (3 out of 109 subjects, 2.8%; p < 0.0001). No contact person developed active tuberculosis during the two-year follow-up. Follow-up data was available for 241 HCWs and 171 HHCs.
While a univariate analysis suggested that the rate of IGRA positivity increased with age in the HHCs and HCWs, this was not confirmed in a multivariate logistic regression analysis (Odds ratio 0.99, 95% confidence interval 0.97–1.02; Supplementary material Table 3). IGRA positivity was independent of the person’s migration background. Among the HHCs, IGRA positivity correlated with the size of a simultaneous TST, while previous positive TSTs as reported by HHCs did not predict a positive IGRA. In the HCWs, IGRA positivity was more likely in persons who reported a positive previous TST (86.5%) than in subjects who reported a negative previous skin test (53.3%; p value <0.0001) (Table 2).
The strongest predictor for LTBI after domestic exposure (Supplementary material Table 3) was a partner with PTB (OR 4.39, 95% CI 1.88–10.26). The univariate analysis suggested that a diseased sibling was also associated with LTBI (OR 3.23, 95% CI 1.51–6.90), but this was not confirmed in the multivariate logistic regression analysis (OR 1.23; 95% CI 0.28–5.36). Sexual contact with the partner increased the prevalence of LTBI from 30.8% (no sex) to 53.3% (sexual activity). Other factors associated with a positive IGRA result among HHCs were a history of previous tuberculosis (4.5 vs 0.3%; p = 0.001), IVDU (13.5 vs 1.2%; p < 0.0001), and alcohol dependency (13.5 vs 1.8%; p = 0.0001).
For the HCWs, exposure to family members with PTB was not associated with LTBI, underlining the focus on professional exposure. Caring for coughing patients (p = 0.02) and a duration of >20 years of occupation (p = 0.004) were associated with LTBI, while the use of respirators, surgical masks, negative pressure rooms or white coats were not (Table 2).
Patients with PTB born in Germany (n = 433) were older than tuberculosis patients born abroad (n = 422) with a mean (±SD) age of 51.8 (±16.2) vs. 42.4 (±15.1) years at diagnosis (p < 0.0001). Uninfected and latently infected HHCs showed a balanced gender distribution but PTB was more prominent in men (n = 568, 66.4%). Foreign-born TB patients were more likely to live in an urban environment (90.7%) than Germany born patients (70.6%; p < 0.0001). Domestic exposure to a diseased partner or child increased the risk of LTBI. In patients with PTB, grandparents (9.1%), partners (8.4%) and fathers (8.0%) were most frequently ill. Children (6.0%) and mothers (5.0%) were less affected.
Former tuberculosis patients had a lower body mass index (BMI) at enrolment than HHCs (mean ± SD, 22.9 ± 5.0 vs 25.2 ± 4.8 kg/m2; p < 0.0001), and 21.2% of tuberculosis patients were underweight. Compared to HHCs, the most prevalent medical risk factors for active disease included alcohol dependency (15.0 vs 5.9%; p < 0.0001), glucocorticoid therapy (7.2 vs 2.0%; p = 0.004), and diabetes mellitus (7.8 vs 4.0%; p = 0.04). Of the diabetic patients, 47% were born outside Germany, mirroring the percentage of foreign-born subjects in the whole cohort (49%). Less prominent risk factors were immunosuppressive treatments, TNF-antagonists therapy, chronic renal failure and HIV infection. IVDU was a strong risk factor for LTBI but was not for PTB.
Fewer patients with PTB were vaccinated with M. bovis Bacillus Calmette-Guérin (BCG) than HHCs (44.6 vs 65.0%; p value <0.0001).
Discussion
We identified additional risk factors for LTBI and active pulmonary tuberculosis among HHCs in Germany, a country of low tuberculosis incidence. Our finding that active PTB was reported more frequently in subjects from an urban environment may be seen as a confounding factor. It reflects the demographic structure of German cities, where immigrants in prospering economic regions and cities contribute 9.8–13.4% of the population, compared to 2.8–6.3% in smaller and rural centres [11]. Active PTB was more frequent in males, underweight individuals, patients on glucocorticoid therapy, diabetic patients, and individuals residing in an urban setting. LTBI was most frequently found among family members of patients with PTB, especially when a partner was affected with whom an active sexual relationship existed. LTBI was more prevalent with alcohol and IVDU. In HCWs, only an exposure of more than 20 years was associated with LTBI, while no impact from measures to prevent transmission was found. Of note, there is no standardised protocol for the prevention of transmission in Germany. Most centres use FFP2 respirators for staff and surgical masks for patients. Some staff use a respirator for more than one occasions, some change after every patient contact. Negative pressure isolation is available in very few centres.
Although follow-up was incomplete with 81.6% of enrolled HCWs and 28.4% of HHCs, no contact person developed active tuberculosis within 2 years after exposure, supporting the poor predictive value of IGRA tests found in other trials [8, 12].
In HCWs, only two risk factors for LTBI were identifiable: caring for coughing patients and a longstanding occupational exposure. This is in line with Indian data from a cohort of nursing students. Christopher et al. found that the duration of caring for tuberculosis patients (OR = 1.10; 95% CI 1.04–1.17) and having performed or assisted in sputum collection (OR = 3.57; 95% CI 1.07–11.84) were both significantly associated with TST conversions [13].
In our study, LTBI was found in almost 40% of staff from tuberculosis wards, exceeding the reported frequency in non-specialised nurses in Germany (5.7–8.3%) and France (15.5%) [14, 15] and Portugal (30.5 %) [15]. LTBI remained even more prevalent than in older non-specialised nurses (>55 years) from Germany (25%) or France (33.3%). Only in Portuguese nurses aged ≥45 years, a prevalence of more than 45% was seen, reflecting the higher prevalence of 25/100.000 in Portugal compared to 5–6/100.000 in Germany and France. Our data indicate that in this well-defined, highly exposed population a migration background has no significant impact on the IGRA status and exposure abroad becomes less relevant.
Our study also provides data on LTBI with implications for domestic contact tracing. Of all positive IGRA test results, 55% were found in contacts exposed to a diseased sibling or partner. Sexual activity with the partner increased the probability for LTBI above 50%. Currently, close monitoring of intimate contact persons is recommended but inquisition about a sexual relationship may refine the focus on individuals at risk [10]. In keeping with a recent Norwegian study [16], our analyses support a tracking strategy involving core family members first and farther contact persons second, complementing current algorithms based mainly on proximity and cumulative exposure time [10, 17]. Despite the poor predictive value of a positive IGRA test for the development of active tuberculosis, core contact persons may require a close follow-up [8].
The data also suggest that outside the domestic setting, contacts with substance dependencies warrant thorough investigations due to their increased risk for LTBI. This is in keeping with earlier data comparing IGRA with TST [18]. However, this finding may be population specific, as IVDU appears not to be a risk for LTBI among US prison inmates [19]. Alcohol consumption is associated with both, higher rates of active tuberculosis and medication induced hepatotoxicity—each requiring particular attention after exposure [20].
Surprisingly, IVDU was not associated with active tuberculosis. The correlation of IVDU and LTBI is known from an observational study from Estonia and Latvia and from an observational study in the United States (adjusted OR = 1.34) [21, 22]. Studies suggesting an association between IVDU and active tuberculosis were mostly small, confounded by HIV-infection or lacking control groups [23–28]. According to our data, IVDU is no independent risk factor for the development of active tuberculosis but rather a surrogate marker for precarious social conditions.
This study shows an increased prevalence of tuberculosis in patients with diabetes mellitus in Germany. While there was no correlation in LTBI, the prevalence of diabetes nearly doubled from 4.0% in HHCs to 7.8% in patients with PTB. This phenomenon was found in both foreign-born and Germany born individuals. Patients with PTB were older than HHCs but a multivariate analysis did not detect an age bias. These data require future evaluation. Until now, the epidemiology was considered to be comparable to neighbouring countries like Denmark or Poland, where no association of tuberculosis and diabetes was found [29, 30]. The relationship is not widely acknowledged by German physicians although evidence is accumulating for various other ethnic groups [31–35].
A limitation of our study is the lack of information on preventive chemotherapy in contact persons. A small number of subjects was offered chemotherapy at the time of recruitment, nevertheless no subject developed active tuberculosis. The poor uptake of preventive chemotherapy has been previously described in Germany [9]. It is associated with the de-centralized management of contact persons who are followed up in the health authority offices of 295 German districts.
Another limitation is that the questionnaires were not verified independently. Some data are based on subjective perception. While recruitment of HCWs and HHCs was prospective, recruitment of patients with PTB was prospective (i.e. patients with active tuberculosis or ongoing antimycobacterial therapy) and retrospective (i.e. patients who were cured from tuberculosis at enrolment). The three cohorts were, therefore, independent and not related to each other. This impairs conclusions about cause and effect of PTB on the identified variables. Although participants were recruited from 18 hospitals and 3 health authorities, the data may not be representative for the whole country. However, given the large number of enrolled individuals, the study provides valid information for risk factors of LTBI and active tuberculosis in a Western European country of low tuberculosis incidence.
In conclusion, our study identified risk groups for LTBI and active tuberculosis based on national epidemiologic data as suggested in the latest global strategy plan for tuberculosis prevention by the WHO. We identified male gender, low body weight, glucocorticoid therapy and diabetes mellitus as risk factors for tuberculosis. Risk factors for LTBI included sexual relationship to a diseased household partner and substance abuse. Among HCWs, only long-term professional exposure to tuberculosis patients was related to LTBI. Despite a high frequency of positive IGRA test results among HHCs and HCWs no contact developed active tuberculosis during a 2-year follow-up period.
Abbreviations
- AFB:
-
Acid fast bacilli
- BCG:
-
M. bovis Bacillus Calmette-Guérin
- BMBF:
-
Bundesministerium für Bildung und Forschung
- BMI:
-
Body mass index
- EEA:
-
European Economic Area
- EU:
-
European Union
- HCW:
-
Healthcare worker
- HHC:
-
Household contact
- IGRA:
-
Interferon-gamma release assay
- IPT:
-
Isoniazid preventive therapy
- IQR:
-
Interquartile range
- IVDU:
-
Intravenous drug use
- LTBI:
-
Latent tuberculosis infection
- OR:
-
Odds ratio
- PTB:
-
Pulmonary tuberculosis
- SD:
-
Standard deviation
- TB:
-
Tuberculosis
- TST:
-
Tuberculin skin test
- UK:
-
United Kingdom
- WHO:
-
World Health Organisation
References
European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe. 2015. www.ecdc.europa.eu. Accessed 26 Oct 2015
World Health Organisation. Framework towards tuberculosis elimination in low-incidence countries [Internet].2014 http://www.who.int/tb/publications/elimination_framework/en/. Accessed 18 Nov 2016
Erkens CGM, Kamphorst M, Abubakar I, Bothamley GH, Chemtob D, Haas W, et al. Tuberculosis contact investigation in low prevalence countries: a European consensus. Eur Respir J. 2010;36(4):925–49.
Hinks TSC, Varsani N, Godsiff DT, Bull TC, Nash KL, McLuckie L, et al. High background rates of positive tuberculosis-specific interferon-γ release assays in a low prevalence region of UK: a surveillance study. BMC Infect Dis. 2012;12:339.
Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, et al. Management of latent mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015;46:1563–76. doi:10.1183/13993003.01245-2015
Fournier A, Antoun F, Larnaudie S. Recent latent tuberculous infection (LTBI) in different sub-groups of contacts. Rev Mal Respir. 2012;29:1079–87.
Ferrara G, Losi M, D’Amico R, Cagarelli R, Pezzi AM, Meacci M, et al. Interferon-gamma-release assays detect recent tuberculosis re-infection in elderly contacts. Int J Immunopathol Pharmacol. 2009;22:669–77.
Zellweger J-P, Sotgiu G, Block M, Dore S, Altet N, Blunschi R, et al. Risk assessment of tuberculosis in contacts by IFN-γ release assays. a tuberculosis network European Trials Group Study. Am J Respir Crit Care Med. 2015;191:1176–84.
Gutsfeld C, Olaru ID, Vollrath O, Lange C. Attitudes about tuberculosis prevention in the elimination phase: a survey among physicians in Germany. PLoS One. 2014;9(11):e112681.
Diel R, Loytved G, Nienhaus A, Castell S, Detjen A, Geerdes-Fenge H, et al. New recommendations for contact tracing in tuberculosis. Pneumologie. 2011;65:359–78.
Bruckner E. Migration und demografischer Wandel [Internet]. Gütersloh: Bertelsmann Stiftung. 2012 p. 1–53. https://www.bertelsmann-stiftung.de/fileadmin/files/BSt/Presse/imported/downloads/xcms_bst_dms_36564_36565_2.pdf Accessed 16 Jun 2016
Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of tuberculosis after recent exposure. a 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med. 2014;190:1044–52.
Christopher DJ, James P, Daley P, Armstrong L, Isaac BTJ, Thangakunam B, et al. High annual risk of tuberculosis infection among nursing students in South India: a cohort study. PLoS One. 2011;6:e26199.
Schablon A, Nienhaus A, Ringshausen FC, Preisser AM, Peters C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the IGRA in German healthcare workers. PLoS One. 2014;9:e115322.
Nienhaus A, Schablon A, Tripodi D, Tripoldi D, Torres Costa J. The prevalence of latent tuberculosis infections among health-care workers—a three-country comparison. Pneumol Stuttg Ger. 2011;65:726–9.
Arnesen TM, Seterelv S, Norheim G, Helgebostad SR, MannsÂker T, Ly IN, et al. Tuberculosis outbreak in Eastern Norway. Tidsskr Den Nor Laegeforening Tidsskr Prakt Med NY Raekke. 2015;135:2160–4.
Lutong L, Bei Z. Association of prevalence of tuberculin reactions with closeness of contact among household contacts of new smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2000;4:275–7.
Grimes CZ, Hwang L-Y, Williams ML, Austin CM, Graviss EA. Tuberculosis infection in drug users: interferon-gamma release assay performance. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2007;11:1183–9.
Weant TE, Turner AN, Murphy-Weiss M, Murray DM, Wang S-H. Can social history variables predict prison inmates risk for latent tuberculosis infection? Tuberc Res Treat. 2012;2012:132406.
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–52.
Morano JP, Zelenev A, Walton MR, Bruce RD, Altice FL. Latent tuberculosis infection screening in foreign-born populations: a successful mobile clinic outreach model. Am J Public Health. 2014;104(8):1508–15.
Rüütel K, Karnite A, Talu A, Abel-Ollo K, Kirvelaite G, Kliiman K, et al. Prevalence of IGRA-positivity and risk factors for tuberculosis among injecting drug users in Estonia and Latvia. Int J Drug Policy. 2014;25(1):175–8.
Healy E, Kelly P, Mulcahy FM, Clancy L. AIDS, i.v. drug use and mycobacterial disease: the Dublin experience. Respir Med. 1992;86(6):491–4.
Poudyal N, Gyawali N, Nepal HP, Gurung R, Pandey S, Amatya R, et al. Risk for developing tuberculosis among intravenous drug users with human immunodeficiency virus (HIV) infection. J AIDS HIV Res. 2014;6(5):104–8.
Rusen ID, Yuan L, Millson ME. Prevalence of mycobacterium tuberculosis infection among injection drug users in Toronto. CMAJ Can Med Assoc J J Assoc Medicale Can. 1999;160(6):799–802.
Sánchez-Carbonell X, Vilaregut A. A 10-year follow-up study on the health status of heroin addicts based on official registers. Addict Abingdon Engl. 2001;96(12):1777–86.
Jansà JM, Serrano J, Caylà JA, Vidal R, Ocaña I, Español T. Influence of the human immunodeficiency virus in the incidence of tuberculosis in a cohort of intravenous drug users: effectiveness of anti-tuberculosis chemoprophylaxis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 1998;2(2):140–6.
Wang W, Xiao H, Lu L. Case-control retrospective study of pulmonary tuberculosis in heroin-abusing patients in China. J Psychoact Drugs. 2006;38(2):203–5.
Masztalerz J, Miller M. The role of diabetes as a factor for increased risk of infection with tuberculosis. Pneumonol Pol. 1990;58:378–85.
Leegaard A, Riis A, Kornum JB, Prahl JB, Thomsen VØ, Sørensen HT, et al. Diabetes, glycemic control, and risk of tuberculosis: a population-based case-control study. Diabetes Care. 2011;34:2530–5.
Marks SM. Diabetes and tuberculosis, US National Health Interview Survey, 2000–2005. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2011;15:982–4.
Walker C, Unwin N. Estimates of the impact of diabetes on the incidence of pulmonary tuberculosis in different ethnic groups in England. Thorax. 2010;65:578–81.
Pablos-MÈndez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health. 1997;87:574–9.
Ferrara G, Murray M, Winthrop K, Centis R, Sotgiu G, Migliori GB, et al. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNFα drugs. Curr Opin Pulm Med. 2012;18:233–40.
Byberg S, Soborg B, Andersson M, Bjerregaard P, Jørgensen ME. Diabetes is a risk factor for tuberculosis in the inuit population of Greenland. Eur Respir J. 2012;40:1289–91.
Acknowledgements
For technical and logistic support, the authors are grateful to F. Daduna, J. Döhling, J. Hofmeister, K. Gaede, A. Glaewe, L. Krabbe (Research Center Borstel), D. Mayer (Department of Medical Microbiology and Hygiene, University of Ulm), S. Jaletzky (Klinik Dr. Hancken, Stade), D. Kraus-Leonhäuser, C. Skrybeck (both Municipal Health Authority, Frankfurt), K. Meywaldt-Walter, G. Reinke (both Municipal Health Authority Hamburg), D. Lehnert (Evanglische Lungenklinik, Berlin), N. Vorreiter (Lungenfachklinik, Immenhausen), J. Paepke (Helios Klinikum Emil von Behring, Berlin), J. Reppe and L. Tittmann (Biobank PopGen, Kiel). PopGen is a member of the popgen 2.0 network (P2 N) which is supported by a grant from the German Ministry of Education and Research (reference 01EY1103).
*Investigators in the TB or not TB consortium in Germany were: M. Allewelt (Evanglische Lungenklinik, Berlin), K. Avsar, A. Neher (Asklepios Fachkliniken München-Gauting, Munich), T. Bauer (Helios Klinikum Emil von Behring, Berlin), S. Blaas (Klinik Donaustauf), M. Fischer (private practice Fürth), S. Ehlers, C. Hölscher, S. Niemann, N. Reiling (all Research Center Borstel), S. Ehlers-Tenenbaum (Thoraxklinik Heidelberg University), P. Hammerl (Lungenfachklinik, Immenhausen), M. von Heinz (Charité University Medicine Berlin), S. Junghanß (Fachkrankenhaus Coswig), S. Kohr (Agaplesion Pneumologische Klinik Waldhof, Elgershausen, Greifenstein), D. Kraus-Leonhäuser, C. Skrybeck (both Municipal Health Authority, Frankfurt), M. Krawczak, W. Lieb (both Christian Albrecht University Kiel), F. Kunitz (Municipal Health Authority, Berlin), B. Lehnigk (Asklepios Nordseeklinik Sylt), V. Leucht (Elbland Reha Großenhain), C.G. Meyer, T. Thye (both Bernhard-Nocht Institut, Hamburg), K. Meywaldt-Walter, G. Reinke (all Municipal Health Authority Hamburg), J. Meywirth (Helios Lungenklinik Diekholzen), M. Mowe (private practice, Iserlohn), R. Muetterlein (Bezirkskrankenhaus Parsberg), M. Pletz (University Hospital Jena), J. Rademacher (Hannover Medical School), C. Priegnitz (Krankenhaus Bethanien Solingen, Solingen), A. Quassem (Lungenfachklinik Lostau), B. Schaaf (Klinikum Dortmund), K.D. Schneider (KRH Klinikum Siloah, Hannover), S. Stenger (University Hospital Ulm), T. Weiß (Helios Klinikum Schwerin).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was carried out by the German Ministry of Education and Research (BMBF, references 01KI0784 and 01KI1007B) funded research consortium on “Pulmonary Tuberculosis–Host and Pathogen Determinants of Resistance and Disease Progression-(TB or not TB)”.
Statements
CL had full access to all of the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis and the content of the manuscript.
Conflict of interest
CH reports personal fees from AstraZeneca, Janssen, Genzyme, Hexal outside the submitted work. RD reports personal fees from Cellestis, Oxford Immunotec, Pharmore and Generium outside the submitted work. TS reports grants from German Ministry of Health during the conduct of the study. CL reports personal fees from Chiesi, Gilead, Abbvie, MSD, Becton–Dickinson, Janssen outside the submitted work. GS, OB, SG, UG, HHU report no conflicting interests.
Role of the sponsor
This study was conducted by the German Ministry of Education and Research (BMBF, references 01KI0784 and 01KI1007B) funded research consortium on “Pulmonary Tuberculosis–Host and Pathogen Determinants of Resistance and Disease Progression- (TB or not TB)”. The sponsor had no influence on design and conduct of the study, nor on data acquisition, interpretation and publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Herzmann, C., Sotgiu, G., Bellinger, O. et al. Risk for latent and active tuberculosis in Germany. Infection 45, 283–290 (2017). https://doi.org/10.1007/s15010-016-0963-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-016-0963-2