Abstract
Arctic regions experience metal pollution, despite their remote location, and the distribution and migration of those metals determine their potential impact on the local environment. Here, a High-Arctic catchment (Revelva, Svalbard) located remotely from human-induced pollution sources is studied with respect to the distribution and migration of chosen trace elements (Ag, Al, As, B, Ba, Bi, Ca, Cd, Co, Cr, Cu, Cs, Mo, Ni, Pb, Sb, Se, Sr, Tl, U, V and Zn) in surface waters. The metal concentrations fluctuated in 2010–2012 between 0.01 and 354 μg L−1, the highest mean-weighted concentration noted for Sr (42.5 μg L−1). The concentrations in the river water were likely influenced by both natural and human-activity-related processes. These factors can produce substances of the same chemical composition (e.g. carbon dioxide, sulphur dioxide and metals may be emitted both by a volcanic eruption and by industrial sources). Therefore, chemometric techniques were used in the current paper to distinguish the multiple sources of pollution in the Revelva catchment. The authors were seeking to determine whether there is indeed evidence for contamination, sufficient to cause environmental damage in polar region. As a result, it was shown that the long-range transport could play an important role in shaping the metal concentration profile of this Arctic tundra environment, capturing both the influence of volcanic eruptions within the region and the human activity in a range of distances from the study site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensification of the industrial activity combined with a limited concern for the environment contributed to the worldwide degradation of the natural environment, including remote areas (Mearns et al. 2015; Zdanowicz et al. 2015; Kadko et al. 2016; Chapman 2016). The direct human impact on the landscape of the Arctic is limited, but remote human activity may change the chemical composition of the elements of the polar environment, especially by introducing contaminants, such as heavy metals (Melke and Uziak 2006; Grotti et al. 2013; Kozak et al. 2013; Hao et al. 2013; AMAP 2005). The migration of heavy metals in the tundra environment requires special attention, since its fragile ecosystem is readily influenced by air, water and soil quality (Pecher 1994). A tundra-covered Arctic river catchment was therefore chosen as a study site, in order to show the pollution levels and the following migration processes in a sequence from source to sink. We aim to establish whether the experienced contamination levels are harmful to the local environment, which can only be done in the context of environmental transport pathways. A suite of interconnected hydrochemical partitioning processes may influence the metal concentrations in the Arctic environment (Fig. 1).
Changes in concentrations may occur already as a result of the duration of atmospheric transport, or as part of a selective deposition process on the surface of glaciers, soil and water. Contaminants, if deposited in surface waters, are directly transported along watercourses. Xenobiotics, i.e. the chemical compounds unknown to the local organisms, metabolised but not needed for their normal functioning, are deposited on the soil surface and migrate via drainage system. Pollutants deposited in the snow cover penetrate with percolating water to the soil and groundwater and drain into surface waters (De Caritat et al. 1996; Niskavaara et al. 1997). Sorption to soil solids could be a major control on the metal concentrations in surface waters. A temporarily elevated load of hydrogen ions in the watercourses, e.g. in the initial phase of snow melt, may cause a sudden acidification of soil and the aquatic ecosystems and result in the release of metals from soils. In brief, it is impossible to determine the full impact of a certain level of contamination in the environment, without the insight into the processes that redistribute them, hence the approach adopted in this paper: to link the concentrations in sources, pathways and sinks.
The final sink of all pollutant deposition is the sea (Fig. 1). It is worth noting that persistent pollutants are deposited in the snow and ice, which then constitute a secondary emission source. Furthermore, the legacy deposition of those can be traced in ice and sediment cores (Mann et al. 2013; Larsen et al. 2010; AMAP 2011). Chemical substances, including chemicals whose production has been banned, are to some extent also trapped in permafrost, and they are gradually released to the environment from there. As a result, the local biota is constantly exposed to their influence (Jörundsdóttir et al. 2014; Ma et al. 2011).
Among polar regions, the Svalbard archipelago is distinguished by its location as the gateway to the Arctic, as well as by its environmental conditions, which make it a pollution reservoir (Ruman et al. 2012). The Revelva catchment, at the Hornsund fjord, has been chosen for a comprehensive study on the deposition of pollutants. This catchment is an ideal study area to recognise sources, transport pathways and deposition processes for the pollution introduced to the Arctic environment globally. The catchments appear to be responding on a time scale of decades, possibly centuries, to changes in metal deposition (Lawlor and Tipping 2003). Surface water, together with precipitation, groundwater and weathering products, represents the environment, as a combination of the natural state and human effects (Vaisanen et al. 1998).
Svalbard Archipelago’s relatively close location to the European Continent makes this sensitive region particularly exposed to the influence of the pollutants, emitted from the industrial European countries (such as the European part of Russia, Norway, Great Britain) (Kozak et al. 2013; Polkowska et al. 2011), as well as the impact of natural-based causes—like forest and agricultural fires (Cahill et al. 2008; Stock et al. 2008; Nawrot et al. 2016), glacial runoff (Bazzano et al. 2014; Stachnik et al. 2016) or volcanic eruption (Iceland) (Langmann et al. 2010; Karasiński et al. 2014; Rose et al. 2006).
Moreover, the landscape of Svalbard, with a prevalence of mountainous areas, favours the accumulation of pollutants transported by the incoming air masses from Europe and Asia. As a result of the processes of wet and dry deposition, the pollutants are delivered to the Arctic ecosystems (Laing et al. 2014; Wojtuń et al. 2013; Laing et al. 2014).
The main aim of this research is to establish the distribution and migration of metals in an Arctic catchment with a minimal influence of local pollution sources. This is achieved by studying concentrations of trace elements in the surface water samples from Revelva, collected in three subsequent years (2010–2012). In this way, both the distribution and the changes of trace elements’ concentrations in the abiotic environment are characterised, providing crucial information for the polar ecosystem protection.
Materials and methods
Study area
The Svalbard archipelago differs from other polar regions due to its specific environmental conditions and geographical location that make the area a sink for pollutants. The catchment of the Revelva river, at the Hornsund fiord, has been selected as the area for complex studies on deposition of pollutants in the polar region due to its location away from potential local emission sources. The Revelva river catchment is supplied both directly and indirectly with atmospheric precipitation, including melting snow cover and the Arie glacier. The region selected creates special environment for assessing sources, transport routes and accumulation of pollutants introduced to the environment. What is more, the Hornsund fiord surroundings are composed of diverse terrain types, connected to the presence of multiple geological formations. Furthermore, the Hornsund fiord has been given a flagship European biodiversity site status, as one of six areas across the continent, in 2002.
The Revelva catchment is located in the Wedel-Jarlsberg Land, in southern part of Spitsbergen island (Svalbard archipelago). The river (Revelva) is fed both directly by atmospheric precipitation and snow melt water streams and a river originating from the Arie glacier. The main watercourse is 5.3 km long (including its part flowing through the lake). The described catchment spans between the ridges of Brategga (645 m asl), Skoddefjellet (733 m asl) and Torbjørnsenfjellet (663 m asl). Revelva drains into the bay of Ariebukta in the south, forming an estuary; tidal waters may reach up to 100 m inland (Radziun 1993). In the upper part of the catchment, the main streams originate from the slopes of Eimfjellet (640 m asl) and Skålfjellet (635 m asl). The catchment is characterised by an asymmetry, with a dominance of left tributaries, of which the proglacial Ariebekken is the largest. The bed of Revelva is majorly paved with pebbles, with occasional boulders and sandy stretches. The rocks eroded and deposited by the river belong to the Precambrian Hecla-Hoek formation, which is composed of Isbjørnhamna group: the formations Ariekammen (shists and marbles) and Skoddefjellet (garnet and mica shists). A small part of the catchment, at the Revelva springs, is built of Eimfjellet lithological group: Gulliksenfjellet quartzite formation, Torbjørnsenfjellet amphibolites and Gangpasset migmatites (Birkenmajer 1990) (Czerny et al. 1993). The Revelva catchment has only a small glacierised part, but past glacial activity has left traces in its upper part in the form of rochees moutonneés in the vicinity of Revelva springs and the basal moraine deposits at the base and on slopes of Skålfjellet. The bottom part of the Revelva valley is an elevated marine terrace, with abrasion stacks. On the terrace, areas of patterned ground and contemporary storm ridges have been formed. At the base of Rotjesfjellet, nival moraines occur, while the larger left tributaries have formed alluvial cones (Matuła et al. 2007). The diversity of the catchment landscape provides an ideal setting for a comprehensive study of processes of pollutant deposition in different parts of the abiotic environment.
Sampling
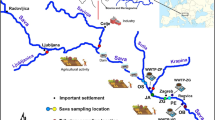
Samples of surface waters (numbers of locations: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15; and 16) and snow (numbers of locations: 17 and 18) were manually collected along the Revdalen valley (Fig. 2), in the points chosen to ensure a representative coverage for a “source to sink” type of study.
Map of the sampled area, where surface water and precipitation samples were taken (based on: Kolondra 2003)
The samples of surface waters were taken with no headspace into air-tight, chemically clean HDPE bottles (the purity of the procedure being verified by daily sample blank collection). Prior to sampling, the bottles were thoroughly cleaned with laboratory glassware cleaning products and multiple times with 18.2 MΩ water (including week-long soaking and removing the water from within the sampling containers four times). During watercourse sampling, the neck of the bottle was directed against the stream in order to prevent the inflow of suspended contaminants, and care was taken so as not to disturb the bed sediments during sampling. Wherever possible, flowing water was sampled from the main stream at depths 20–50 cm below water level (if not, then at one-third of the total depth).
At the same time as the surface waters, we collected also snow samples from the uppermost snow cover layer (top 15 cm), into clean bags and with single-use, sterile sampling devices; they were stored frozen until laboratory analysis. These samples represented the remaining part of the accumulated winter snowfall and hence can only be used as a background information here. Because of the diversity of the components found in water samples, and the changes inside the samples which can take place under the impact of physical and chemical, photochemical and biochemical processes, it is extremely challenging to ensure that the sample has kept its natural, original composition. In order to limit the unwanted influence of the above-mentioned occurrences, as much as possible, the chemical analysis was conducted in the shortest possible time. In addition, measures were taken to guarantee the sample composition stability (both chemical and physical) during the time of the transport and storage, up to the time of conducting the analysis. For transportation, all samples were stored in sealed plastic containers, in the dark and below 4 °C. Before analysis to determine metals, the samples were filtered through 0.45-µm filters and were acidified by HNO3 (ultrapure).
A total of three summer seasons are represented in this study (2010–2012). All samples were transported back to the laboratory in Gdańsk University of Technology, Poland, and analysed immediately after shipping. Every summer season 15 samples were picked from various points, situated along the Revdalen Valley, spreading from the source of the river Revelva to its mouth flowing directly into the fjord, passing through the Revvatnet lake. Samples were collected during a single day, within a fixed period each year (the first week of July), as this was the time of the biggest probability to sample from all the measuring points (avoiding the times when parts of the river were frozen or tributaries dry). On each of the sampling days, a blank was collected and then analysed in identical conditions as the other samples.
Laboratory analysis
Milli-Q deionised water was used during the determination of the various target analyte groups and to prepare sample container blanks. The concentrations of organic and inorganic compounds (metals and non-purgeable organic carbon (NPOC), as well as pH and electrical conductivity (EC), were determined in precipitation and surface water samples by inductively coupled plasma mass spectrometry (Thermo Scientific XSERIES 2 ICP-MS Germany, Collision Cell Technology, Cool gas flow Ar: 12 l/min, Cell gas flow He/H: 5.5 ml/min), the Analyzer TOC-VCSH/CSN SHIMADZU, and the electrochemical measurements in the field (Microcomputer pH-metre/O2-metre CX-401 by ELMETRON: electrode-type ESAgP-301 W and EC-metre OK.-102/1, RADELIKS). The NPOC analyses had measurement range (MR), limit of detection (LOD), limit of qualification (LOQ), and coefficient of variation (CV) (in mg L−1) as follows: 0.03–10, 0.030 and 0.10. The CV of NPOC analyses ranged from 0.1 to 2.0 %. The MR, LOD and LOQ (in μg L−1) for metals were at three different levels. The first group (Be, Ga, Rb, Cd, Cs, La, Ir, Tl, Th and U) had an MR of 0.01–1000, LOD of 0.01, and LOQ of 0.030, respectively. Metals such as Li, Al, V, Cr, Mn, Co, Ni, Cu, Zn, As, Ba, Pb had MR, LOD, and LOQ at 0.1–1000, 0.1, and 0.3, respectively. Finally, Sr and Bi were characterised by MR, LOD and LOQ of 1.0–1000, 1.0 and 3.0, respectively. Metal concentrations CV ranged from 0.5 to 1.5 %.
Quality assurance/quality control (QA/QC)
The analytical procedures applied in the determination of individual components in environmental samples with various matrix compositions should be validated against certified reference materials. The data obtained in the research were subjected to strict quality control procedures. Prior to measurements, the pH electrode three-point calibration with temperature compensation was performed. For electrode calibration, buffer solutions (traceable to SRM from NIST and PTB, pH 4.01 (phthalate), pH 7.00 (phosphate), pH 9.00 (borate), pH 4.01/pH 7.00/pH 9.00 (25 °C) Certipur®) of MERCK Millipore were used. The analysis of metals involved the application of Standard Reference Material NIST 1643e Trace Elements in Water, and Reference Material Enviro MAT ES-L-2 CRM, ES-H-2 CRM SCP SCIENCE. The calibration of the apparatus was based on reference materials by Inorganicventures ANALITYK- CCS-4, CCS-6, CCS-1, IV-ICPMS-71A. Potassium hydrogen phthalate by Nacalai Tesque (Japan) was used for the calibration of the TOC analyser. The sensitivity of the applied methods was tested by injecting standard mixtures of the analytes in the measurement range concentration. Linear calibration curves of the peak area against standard concentration showed correlation coefficients (R 2) in the range of 0.898–0.999 for all standards. Each sample was analysed in triplicate. During the analysis of the samples, procedural blanks (Milli-Q water with no certified material addition) were prepared for every batch of six samples to check the instrumental background.
Cluster analysis (CA) and principal components analysis (PCA)
Cluster analysis (CA) is a well-known and widely used data mining approach for various purposes with its hierarchical and non-hierarchical algorithms (Massart and Kaufman 1983; Einax et al. 1997). In order to cluster objects characterised by a set of variables (e.g. sampling sites by chemical concentrations), one has to determine their similarity. A preliminary step of data scaling is necessary (autoscaling or z-transform) where normalised dimensionless numbers replace the real data values. Thus, even serious differences in absolute values are reduced to close numbers. Then, the similarity (or more strictly, the distance) between the objects in the variable space has to be determined. Very often the Euclidean distance is used for clustering purposes. Another way of measuring similarity is calculation of the correlation coefficient between the objects. Thus, from the input matrix (raw data) a similarity matrix could be constructed. There is a wide variability of hierarchical algorithms of objects linkage, but the typical ones include the single linkage, the complete linkage and the average linkage methods. The representation of the results of the cluster analysis is usually performed by a tree-like scheme called dendrogram comprising a hierarchical structure (large groups are divided into small ones). The hierarchical methods of clustering mentioned above are called agglomerative.
Principal components analysis (PCA) is a typical display method, which allows to estimate the internal relations in the data set. There are different variants of PCA but basically their common feature is that they produce linear combination of the original columns in the data matrix (data set) responsible for the description of the variables characterising the objects of observation. These linear combinations represent a type of abstract measurements (factors, principal components) being better descriptors of the data structure (data pattern) than the original (chemical or physical) measurements. Usually, the new abstract variables are called latent factors, and they differ from the original ones named manifest variables. It is a common finding that just a few of the latent variables account for a large part of the data set variation. Thus, the data structure in a reduced space can be observed and studied (Massart et al. 1998).
Generally, when analysing a data set consisting of n objects for which m variables have been measured, PCA can extract m principal components PCs (factors or latent variables) where m < n. The first PC represents the direction in the data, containing the largest variation. PC 2 is orthogonal to PC 1 and represents the direction of the largest residual variation around PC 1. PC 3 is orthogonal to the first two and represents the direction of the highest, residual variation. The projections of the data on the plane of PC 1 and PC 2 can be computed and shown as a plot (score plot). In such a plot, it is possible to distinguish similarity groups. According to the theory of PCA, the scores on the PCs (the new co-ordinates of the data space) are a weighted sum of the original variables (e.g. chemical concentrations):
where Y is an indication of the variable value (e.g. concentration) and γ are the weights (called loadings). The information hidden in the loadings can also be displayed in loadings plots. It is important to note that PCA requires very often scaling of the input raw data to eliminate dependence on the scale of the original values.
Modelling of dissolved heavy metal speciation
In order to determine species the occurrence of metal species, a chemical speciation modelling was performed using PHREEQC software coupled to the MINTEQ database supplemented with chemical reactions of Mo from LLNL database (Parkhurst and Appelo 2000). Input data consisted of water temperature (assumed at 5 °C), pH and the chemical composition of water. We assumed water temperature of 5 °C that is close to mean air temperature for August observed nearby in Polish Polar Station in Hornsund. Water temperature reported by Marszałek and Wasik (2013) for streams at the Revelva river was found similar to air temperatures in the Polish Polar Station (Wawrzyniak—personal comm.).
Results and discussion
The concentrations of trace elements (Ag, Al, As, B, Ba, Bi, Ca, Cd, Co, Cr, Cu, Cs, Mo, Ni, Pb, Sb, Se, Sr, Tl, U, V, Zn) and NPOC, as well as the pH and EC of water samples from the surface waters of Revelva catchment, are reported in Table 1.
The concentration of Cu was stable in the years 2010–2011, while in 2012 it showed a sharp decrease. A clear rise was observed in the concentrations of Cd, Pb, Cr, Ni and Al, while the opposite direction of change was found for Mn and Mo. In the years 2011–2012, the Cs and Th concentrations in the analysed samples did not exceed LOD. The concentration of U and Sr in 2010–2011 was distinctly increasing, while in 2012 there was marked decrease. The sum of metals in the chosen sampling points (Fig. 3) was distinctly higher in 2010 and 2011 than in 2012. In the samples taken from the spring in the upper part of Revelva catchment, in the years 2010–2011, a ten times higher concentration of metals was noted than in the same period in the Revvatnet lake waters or the Revelva estuary.
The elevated concentrations of metals in the samples from 2010 to 2011 could have been influenced by volcanic eruption in Iceland. The high concentration of metals in the spring might be an indicator of the fast elution of contaminants deposited from the long-range atmospheric transport on mountaintops and glacier surfaces. The increase in the total metal content in 2012 along the Revelva river (in the order: springs < lake < estuary) suggests accumulation and then gradual elution of contaminants of particular components of the environment, where the final sink of the metal deposition is Hornsund fjord. Since the polar ecosystems are highly susceptible to pollution-induced degradation (due to their simple trophic structure and low biological activity rates), the low concentrations of contaminations considered here may cause changes in tissues and organs. The possibilities to maintain homoeostasis (by natural assimilation or neutralisation of contaminants) in the Arctic are very small. Therefore, the comparison of pollutant concentration with other regions of the world is not directly informing about the influence of a particular substances in the local environment (Kozak et al. 2013).
The variability of pH and EC was also observed in the Revelva catchment. The wide span between the maxima and minima of these parameters in the subsequent years was likely due to the dynamic behaviour of the tributaries, e.g. the proglacial streams. The temporal variability of mean values was less pronounced, and the average values of pH and EC were uniform across the three investigated summer seasons.
Geochemical and precipitation sensitivity coefficient indices
The geochemical indices are presented here, showing the sensitivity of surface water pollution to the influence of precipitation, and their relation to the geochemical background of the arctic tundra environment. This resulted in an assessment of the impact of precipitation (snow) on pollutant deposition. The geochemical background investigation and the calculation of geochemical indices are tools enabling the evaluation of the environmental pollution in the study site (Al-Haidarey et al. 2010; Chakravarty and Patgiri 2009; Loska et al. 2004; Reimann and De Caritat 2005). The geochemical background is the natural range of concentration for a given element, while the anomalies from this level may be induced by both natural processes and human activity. However, its determination is a complex task due to the variety of factors influencing the naturally occurring levels of trace element concentrations; sometimes both the natural and human impacts are simultaneous and cause a mixed anomaly. All the described anomalies may cause pathological changes in the biota of the fragile polar ecosystems.
In this study, the contamination factor (CF) was used to evaluate the degree of contamination in water samples by comparison of a particular element concentration in the given sample and:
-
the precipitation as snow (precipitation sensitivity coefficient index (CF) (\(\mathop C\limits_{\text{snow}}\)))
-
the lowest concentration of the analysed element (noted with the use of the statistical method Q1)
\(CF = {\raise0.7ex\hbox{${\mathop C\limits_{0 - 1} }$} \!\mathord{\left/ {\vphantom {{\mathop C\limits_{0 - 1} } {\mathop C\limits_{{{\text{snow}}/{\text{quartile}}}} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\mathop C\limits_{{{\text{snow}}/{\text{quartile}}}} }$}}\) where \(\mathop C\limits_{0 - 1}\)—arithmetic mean of the analysed element concentration (in the surface water samples)
\(\mathop C\limits_{\text{snow}}\)—the concentration of the analysed element in the atmospheric precipitation snow samples
\(\mathop C\limits_{\text{quartile}}\)—the lowest concentration (the average value of the concentrations below the first quartile) of the analysed element (in the surface water samples) (Al-Haidarey et al. 2010; Chakravarty and Patgiri 2009; Loska et al. 2004; Reimann and De Caritat 2005).
By summing up contamination factors for all analysed elements, the degree of contamination was calculated (C deg), which enabled the classification of the area into one of the four categories: insignificant contamination (CF < 1 or C deg < 8, marked with yellow in Table 2), moderate contamination (1 ≤ CF < 3 or 8 ≤ C deg < 16, marked with green in Table 2), significant contamination (3 ≤ CF < 6 or 16 ≤ C deg < 32, marked with blue in Table 2), and heavy contamination (CF ≥ 6 or C deg ≥ 32, marked with red in Table 2).
With respect to the CF calculated here, the highest influence on the contamination of the study area had Al, Pb and Mn (CF > 6), Cr and Mo had a significant influence (3 ≤ CF < 6), and the elements Zn, Rb, Th and U had moderate impact (1 ≤ CF < 3) (Table 2A). It should be emphasised that the enrichment of rivers relative to the precipitation is also linked to rock weathering.
Based on the calculations of Cdeg for separate sampling seasons (Table 2A), a change in the state of the environment was observed, as C deg shifted between significant contamination in 2010 (C deg = 18.8) or 2012 (C deg = 16.7) to heavy contamination in 2011 (C deg = 36.6). The values of CF indicate the possible anthropogenic source of the metals Cr, Zn and Pb. Because of the lack of settlements within or in the direct vicinity of the sampling area, a likely source of contaminants in surface waters is the long-range transport, ship traffic or alternatively a sudden release of stored contaminants in snow, sediments or soils. In Table 2B, we show the contamination factor (CF) and the degree of contamination (C deg), calculated for chosen elements in surface waters sampled in 2010–2012. Based on the analysis of the pollution-level factors in the study site, we observe an increase in pollution level in the years 2010–2011, while in 2012 a decrease was noted. An increase in the years 2010–2011 can be described by such elements such as Cr, Mo, Mn, U and Zn, Sb, Sr. Interestingly, in 2012 the concentration of most of the elements decreased to the level described as “average pollution”, which can be treated as a natural background for the metal content. Zn and Pb are an exception here—it was shown that the levels of those elements are very high. An additional factor worth considering is the occurrence of two major volcanic eruptions in Iceland the years 2010–2011, which could influence the trace element supply to Svalbard via dust deposition from long-range transport (Langmann et al. 2010; Karasiński et al. 2014; Tepe and Bau 2014).
Comparing the given results of the pollution level to the precipitation sensitivity factors, we observed a similar tendency in the changes of some metal concentration levels in surface waters. At the turn of 2010 and 2011, an increase in the concentration of elements such as Cr, Mo, Mn, U and Zn was marked. Furthermore, a decrease in most metals concentration, with the exception of Zn and Pb (which were characterised by high concentration levels), was noted. Precipitation and rock weathering likely played a major role in the process of metal supply into the catchment area, since they originate from the atmosphere infused with gaseous pollutants, dusts and aerosols of both natural and artificial (anthropogenic) origin. The mineral components may both be supplied from atmospheric deposition and be rinsed into surface waters from glaciers and the tundra vegetation.
In the evaluation of the environmental impact of a given element, it is important to recognise its speciation. This determines its potential for harmful impact on the abiotic environment and the biosphere. Elements such as Al, Mn, Pb, Cr, Mo, U had highest impact shown in CF ranging from 6 to 171, whereas CF in group of elements including U, Sb, Sr, Rb, Zn ranged from 2 to 3. Elements such as Th, Cu, Ni, V featured with CF lower than 1. In acidic conditions, many metals are transferred into the solution in ionic form (e.g. Pb, Cd, Cu and Al) and their migration is also dependent on their preference for forming coordination complexes. Also, the increasing ionic strength of the solution induces intensive formation of metal complexes in water. All these processes would therefore contribute to the potential harmful impact of the water on the biotic environment, free ions being more toxic than metal complexes, while the inorganic complexes being more harmful than their organic counterparts. The toxicity of a metal compound is not only dependent on the metal ion (e.g. whether it belongs to heavy metals group), but also on the ligand type (e.g. whether it is humic or anthropogenic compounds). In particular, surfactants are catalysing the processes of complexing in surface waters. Moreover, the presence of the compounds lowering the surface tension (surfactants), used popularly and on a large scale, leads to the increase in the solubility of heavy metals in water ecosystems (Jensen 1999; Holt 2000). Compounds from the surfactants group released to the atmosphere are present in the vapour phase as aerosols and can be adsorbed on the surface of the particulates. Along with dry and wet deposition, they can be transported on long distances, reaching the pristine regions of the Arctic.
Chemometric analysis
During the first stage of the chemometric analysis, a diagram (Fig. 4) was drawn to picture the relationships between the variables. In Fig. 4, the correlation coefficients of chosen variables are shown. A high correlation coefficient (1–0.8) was found between the following elements: V/Sr; V/Mo; V/Cr; Sr/Cr; Sr/Mo; Mo/Cr.
In the analysis of a particular element sources, it is helpful to compare their concentrations to the NPOC concentration. The leaching of particular elements by the environmental waters besides the solubility parameters, adsorption and reactions with organic compounds may change the concentration of a given metal (Greenwood et al. 2013). The limited removal of heavy metals results in their accumulation in surface water bodies. The presence of organic matter (measured as NPOC here) in waters changes the hydrogeochemical cycles of trace elements by means of redox reactions, peptisation and sorption. However, here we found no significant positive correlation between the concentrations of trace elements and NPOC, whereas stronger positive correlations were found between particular metals concentrations. In particular, V, Sr, Ba and Mo were correlated with other metals. A probable reason for this may be the composition of the local geological substratum, as trace elements may be co-eluted from polymetallic ore minerals. We normalised concentrations to Al or Sc to account for lithogenic background. A likely source of Pb in the Revelva catchment is the long-range atmospheric transport of pollutants as well as the polymetallic ores in the local substratum. The ore-processing smelters are Cu–Ni factories of the Kola peninsula (Nuttall 2005). Other metals may also be supplied from industrial sources located in a distance from the study site in particular from past and present mining or metallurgy, as well as from dispersed sources such as various combustion processes and traffic (including ships). The moderately volatile heavy metals and those absorbed onto suspended particles are transported at long distances, polluting the remote Arctic (Gong and Barrie 2005).
The main reason to use PCA was to analyse a data set composed of multiple interconnected variables in order to distinguish a possibly small number of independent factors. The analysis encompassed a creation of a new coordinate system based on the independent variables (principal components), of which the first few contain most the information about the original variables. For this purpose, an eigenvector database of the concerned space was used (Fig. 5).
As a result of the PCA, four principal components were distinguished. The first of those (PC1) enabled the explanation of 39.5 % of the total variability, and it described the trace element content originating from the bedrock. It can be assumed that the common source of the elements (V, Cr, Cu, Sr, Ba, Mo) with significant factor loadings is rock weathering. A significant quantity of those metals is delivered to surface water reservoirs in the watercourses flowing from the nearby mountains and glaciers. A high correlation coefficient between those metals and their high factor loadings in PC1 describes the natural process of rock weathering and the leaching of minerals. The second latent component (PC2) explains 14.9 % of the total variance and describes the significant correlation of Al, Rb and Se. It could be assumed that these metals, as components of the Earth’s crust, could come from geological processes, resulting from endogenic factors, including volcanism. The third principal component (PC3) explains 10.5 % of the total variability and is characterised by high factor loadings of the following parameters: NPOC, pH and specific electric conductivity. It can be assumed that one of their sources may be the organic matter decomposition processes, as well as wet atmospheric deposition augmenting the concentration of organic compounds and salts, which leads to pH changes as well. The last component (PC4) enables explanation of 7.6 % of the total variance and indicates the impact of the As content. This element has two opposite sources: natural processes (volcanic eruptions, sedimentary rock dissolution) and human activity (fossil fuel burning, metallurgy and the exploitation of non-metallic ores).
While PCA aims to reduce number of variables maintaining maximum amount of information, cluster analysis (CA) is a tool for exploratory data analysis that orders elements of the group according to their maximum similarity to one another (Fig. 6). The graphic presentation of the similarity is the hierarchical dendrogram.
As a result of the CA, two major clusters are formed and in each one of them two subclusters could be interpreted. The first major cluster with two subclusters was formed by the elements originating from bedrock and the geological processes. Their common denominator was the rock weathering and dissolution. The second major cluster could also be subdivided. In this case, Pb and Zn differ from As and other water parameters (conductivity, acidity and alkalinity). It could be related to human activity impact (long-range transport and industry, including mining) on the Arctic tundra environment.
Figure 7 shows the results of grouping the sampling locations by factor scores in the study years. Additionally, on the plot the relationship between the chemical parameters and the sampling events groups is shown. In principle, it is possible to find out which parameters are responsible for the formation of the sampling locations groups. The sampling events are temporally separated.
A clear difference with respect to the year of sampling was observed. An outlier [15-2010] (number of sampling site and year) was also present. Interestingly, in 2010 an enormous volcanic eruption took place in Iceland, which most probably influenced the detected levels of trace elements in the Revelva catchment and the outlier formation.
Analysis of the contaminant influx into the Hornsund fiord area based on the distribution of backward trajectories
A backward trajectory reproduces the history of air mass movement, based on 3D numerical transport models, and provides a tool for the analysis of transport and dynamics of atmospheric pollution. The backward trajectories were computed with Hybrid Single-Particle Lagrangian Integrated Trajectory Model (HYSPLIT) version 4 (Draxler 1999), based on Global Data Assimilation System meteorological data set. Figure 8 shows the effect of clustering of backward trajectories for the Hornsund fiord area, during the measurement periods of 2010, 2011 and 2012. The trajectories were calculated for 1000 m agl starting point with 3-h interval and than clustered using Ward’s method (Stunder 1996).
In the years 2010–2012, Hornsund was typically influenced by air masses from northern Russia, eastern Europe Iceland, Greenland and northern Canada. In 2010, the air mass trajectory distribution was multimodal with high number of air masses coming from the north (over 35 %). This year represented also a pattern of shorter transport distances than in the following two years. In 2011 and 2012, an additional important area of air mass origin was Canada (about 24 % of air masses each year). It is also worth noting that in 2011 large number of trajectories crossed over Iceland. This might be the reason for higher metal content concentrations, which coincidences with Grimsvotn eruption. Most characteristic feature for year 2012 is high number of air masses originating from northern Russia (24 %) and relatively high influx of air from eastern Europe (16 %)
Heavy metal speciation
Elements having high average CFs during study period (Al, Mn, Pb, Cr, Mo, Zn and U) have been chosen for a speciation modelling in PHREEQC software.
The speciation of Al, Mn and Pb including their toxic forms is dependent of the pH of water (Figure S1, S2). In the case of Al, a distinctive influence of the lowering pH on the increase in the concentration of toxic forms of this element, i.e. Al3+ and Al(OH) +2 , was shown (Gensemer and Playle 1999). In the case of seasons 2011–2012, the proportion of toxic forms of Al was much higher than in 2010, when its low level was concordant with a considerably higher pH value. In the same year, a high proportion of coordination complexes (i.e. HAlO2 AlO -2 ) was also noted. The majority of Pb2+ and Mn2+ ions content in the measurement period was their toxic forms (Figure S2), and the influence of pH on the change in their speciation was insignificant. Only in the case of pH exceeding 7.5, there was a slight decrease in Pb2+ concentration observed. Despite that the proportion of Pb complexes (i.e. Pb(OH)2 and PbOH+) as well as Mn complexes (Mn(OH)2 and Mn2(OH) +3 ) was much lower in comparison with the simple ionic forms, the high proportion of Mn2+ in the total Mn concentration was also observed in the forested catchments of Finland (Nystrand et al. 2012). In the Arctic Revelva catchment, a further pH decrease would induce the increase in toxicity of the dissolved Al, while the influence of pH on Pb and Mn toxicity is limited.
The variability of speciation forms of Cr and Mo was characterised by a strong dependence on pH (Figure S3). The highest concentration of Mo was observed in 2010 and was decreasing until 2012. An opposite effect was observed for Cr3+, whose concentrations were higher in 2011–2012 than in 2010. The main forms of Cr were Cr3+ and Cr(OH)+2 below the pH of 6, while above this value forms with multiple OH groups dominated (i.e. Cr(OH)3 and Cr(OH) +2 ). In the years 2011–2012, the lower pH could have influenced the higher concentration of the toxic forms of Cr. The lowering pH in the years 2010–2012 could have also influence the drop in Mo concentration.
The variability of concentrations of Zn and U with changing pH of water is presented (Figure S4). The dominant toxic form of Zn in the measured pH range was also the ionic one (Zn+), but with increasing pH the proportion of complex forms (i.e. Zn(OH)+ and Zn(OH)2) was rising. In general, the role played by the metal complexes in reducing environmental toxicity is small in the study area. Uranium occurred in the form of metal complexes and above the pH of 6.5 those included –OH group (i.e. UO2OH+). For pH < 6.5, the dominant form of U was UO2 2+. The potentially most toxic metal of the four described in this paragraph is Zn, showing the highest proportion of ionic forms in the total concentration. The U occurs as coordination complexes more frequently.
Conclusion
The Arctic Revelva river catchment in the summer seasons of 2010, 2011 and 2012 was characterised by highly variable concentrations of highly toxic trace elements: Cd, Pb, Cu, Zn and Cr. Differences in metal concentrations between years, and temporal variations in individual water types, can be explained qualitatively in terms of mineral surfaces, complexation, chemical weathering and sorption to solid-phase soil organic matter. The likely influence on these concentrations is a mixture of natural processes and human activity located far away from the catchment (Bazzano et al. 2014). In particular, a natural source of metals could be the volcanic eruptions in Iceland (Karasiński et al. 2014; Tepe and Bau 2014). Karasinski et al. (2014) has indeed shown that the transport of volcanic dusts and aerosols towards Svalbard has occurred in both 2010 (from the Eyjafjallajokull eruption at 63.63°N 19.62°W) and 2011 (when Grimsvotn erupted at 64.42°N 17.33°W). The volcanic products may include adsorbed organic and inorganic metal compounds, which would also be deposited after long-range transport in Svalbard. The elevated metal concentrations in the surface waters of Revelva catchment were likely resulting from volcanic dust and aerosol deposition, as there occurred a distinct shift towards lower metal concentrations and seasonal mean EC values between the two seasons of 2010–2011 and the 2012 summer, when no volcanic activity in the vicinity of Spitsbergen was noted.
Comparing concentrations measured in Revelva catchment to published data sets (Headley 1996; Ahn et al. 2009; Banks et al. 1998; Drbal et al. 1992), we can observe that our results are relatively higher, which can indicate the impact of the volcanic eruptions on the distribution of metals in the Arctic environment, considering the coinciding periods of sampling and volcanic episodes. Zinc, Mn, Cr and Pb concentrations in surface water in our studies were higher compared to other sites in Svalbard (Drbal et al. 1992; Banks et al. 1998). Also, Zn and Pb concentrations in precipitation were higher in our studies compared to other studies on precipitation in Svalbard (Ahn et al. 2009; Headley 1996). Furthermore, this study is a contribution to the relatively sparse research on the migration of metals in the Arctic surface waters, and the complex relations between human activity and natural pollution sources. One of the most intensive natural sources of pollution, which was also noted in Svalbard, is volcanic eruption (Burzyk et al. 2004). They cause gaseous emissions to the atmosphere (in particular of water vapour, sulphur dioxide, hydrogen sulphide, carbon dioxide, hydrogen chloride and fluoride), as well as the emissions of dust particles and aerosols (mixtures of organic compounds, enriched in metal particles). The dust particles may remain suspended in the atmosphere for long periods and be transported for long distances (Robock 2000; Mather et al. 2003). Weather conditions play a crucial role in this movement of contaminants. Horizontal displacement of air masses favours dispersion of contaminants, resulting in the decrease in their concentration. On the other hand, the downward movement of the polluted air accelerates the deposition of particulate matter. Also, the steady air masses enable free fall of particles into the lower layers of the atmosphere. The volcanic ashes may form a contaminated layer in the stratosphere. During large eruptions, this layer may cause disturbance of the ecological balance and the climatic system (Glasow et al. 2009).
Moreover, the colonies of seabirds and mammals play an important role in introducing heavy metals and microelements to the Arctic tundra ecosystems (Godzik 1991; Wagemann et al. 1995; Brimble et al. 2009). The airborne transport of pollutants may be happening along single-hop or multi-hop paths. The single-hop pathway covers emission of the chemical compound to the atmosphere, its transport and subsequent deposition on the Earth surface with no return to the atmosphere. Acids and heavy metals (except of mercury) behave in this manner. A volatile chemical may return to the atmosphere after its deposition on the Earth surface and keep transferring further in the environment, performing further “hops”. This phenomenon is called ‘the grasshopper effect’ (Fernández and Grimalt 2003). In the recent years, a concern has risen in the transport and fate of pollutants in polar areas, which are remote from industrial centres. For example, bioaccumulation of heavy metals has increased in mosses and soils of SW Svalbard since 1990s (Wojtuń et al. 2013). The novel analytical techniques have enabled the conclusion that the Arctic, which was in the past considered pollution-free, became an area of pronounced human impact, and the long-distance transport of pollutants has been recognised as a threat to the polar regions. However, the exact impact of this phenomenon is impossible to assess without the deposition and migration studies in the Arctic catchments.
The sources and impact of the trace element concentrations in the surface waters of Revelva catchment were also analysed by means of geochemical indices calculation. In particular, the contamination factor compared the levels of trace metal concentration, showing that human impact on the surface water quality in the study site is very likely. There is a high likelihood that volcanic eruptions heavy metals concentration levels presented in this study. The analysis of the degree of contamination supports this assertion.
The recognition of the processes of pollutant distribution in the atmosphere is an issue of top priority. Thanks to this it will be possible to learn about mechanisms that affect the pollutants on a global scale. We have tracked the changes in trace element concentrations in particular elements of the water cycle within an Arctic catchment, from atmospheric precipitation through springs, rivers and lakes. As a result, we provide a likely characteristic of a toxic metal compound cycle in such a setting. The concentration of a particular element undergoes multifaceted change, which can only be recognised in long-term detailed monitoring efforts. The particularly important influences on the surface water pollution are the accumulated effect of winter air mass inflow (contributing human-induced, but also volcanic contamination) and the local geological substratum.
References
Ahn LY, Ji P, Park H (2009) Metal accumulation in sea urchins and their kelp diet in an Arctic fjord (Kongsfjorden, Svalbard). Mar Pollut Bull 58:1571–1577
Al-Haidarey MJS, Hassan FM, Al-Kubaisey ARA, Douabul AAZ (2010) The geoaccumulation index of some heavy metals in Al-Hawizeh Marsh, Iraq. E-J Chem 7:157–162
AMAP (2005) AMAP Assessment 2002: heavy metals in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. (first published as electronic document in 2004)
AMAP (2011) Snow, Water, Ice and Permafrost in the Arctic (SWIPA): Climate Change and the Cryosphere. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. (first published as electronic document in 2013)
Banks D, Sletten RS, Haldorsen S, Dale B, Heim M, Swensen B (1998) The thermal springs of Bockfjord, Svalbard: occurrence and major ion hydrochemistry. Geothermics 27:445–467
Bazzano A, Rivaro P, Soggia F, Ardini F, Grotti M (2014) Anthropogenic and natural sources of particulate trace elements in the coastal marine environment of Kongsfjorden, Svalbard. Mar Chem 163:28–35
Birkenmajer K (1990) Hornsund Spitsbergen geology 1:75000, Map and comment, (in polish) University of Silesia. Katowice, Poland
Brimble SK, Blais JM, Kimpe LE, Mallory ML, Keatley BE, Douglas MSV, Smol JP (2009) Bioenrichment of trace elements in a series of ponds near a northern fulmar (Fulmarus glacialis) colony at Cape Vera, Devon Island. Can J Fish Aquat Sci 66:949–958
Burzyk J, Zjawiony I, Burzyk M, Głowacki P (2004) Changes of heavy metals’ concentrations in the surface waters of SW Spitsbergen as an effect of volcanic eruptions in subpolar zone. Ecohydrol Hydrobiol 4:27–33
Cahill CF, Cahill TA, Perry KD (2008) The size- and time-resolved composition of aerosols from a sub-Arctic boreal forest prescribed burn. Atmos Environ 42:7553–7559
Chakravarty M, Patgiri AD (2009) Metal pollution assessment in sediments of the Dikrong River. N.E. India. J Human Ecol 27:63–67
Chapman PM (2016) Toxicity delayed in cold freshwaters? J Great Lakes Res 42:286–289
Czerny J, Kieres A, Manecki M, Rajchel J (1993) Geological Map of the SW Part of Wedel-Jarlsberg Land, Spitsbergen. In: Manecki A (ed) Institute of geology and mineral deposits. University of Mining and Metallurgy, Kraków, Poland
De Caritat P, Reimann C, Äyräs M, Niskavaara H, Chekushin VA, Pavlov VA (1996) Stream water geochemistry from selected catchments on the Kola Peninsula (NW Russia) and in neighbouring areas of Finland and Norway: 2, Time-series. Aquatic Geochem 2:169–184
Draxler RR (1999) HYSPLIT4 User’s Guide. NOAA Tech. Memo. ERL ARL-230, NOAA Air Resources Laboratory, Silver Spring
Drbal K, Elster J, Komárek J (1992) Heavy metals in water, ice and biological material from Spitsbergen. Svalbard. Polar Res 2:99–101
Einax J, Zwanziger H, Geiss S (1997) Chemometrics in environmental analysis. J. Wiley, Weinheim
Fernández P, Grimalt JO (2003) On the Global distribution of persistent organic pollutants. Chimia 57:514–521
Gensemer RW, Playle RC (1999) The bioavailability and toxicity of aluminum in aquatic environments. Crit Rev Environ Sci Technol 29:315–450
Glasow R, Bobrowski N, Kern Ch (2009) The effects of volcanic eruptions on atmospheric chemistry. Chem Geol 263:131–142
Godzik B (1991) Heavy metals and macroelements in the tundra of southern Spitsbergen: the effect of little auk Alle alle (L.) colonies. Polar Res 9:121–131
Gong SL, Barrie LA (2005) Trends of heavy metal components in the Arctic aerosols and their. Sci Total Environ 342:175–183
Greenwood PF, Brocks JJ, Grice K, Schwark L, Jaraula CMB, Dick JM, Evans KA (2013) Organic geochemistry and mineralogy I characterization of organic matter associated with metal deposits. Ore Geol Rev 50:1–27
Grotti M, Soggia F, Ianni C, Magi E, Udisti R (2013) Bioavailability of trace elements in surface sediments from Kongsfjorden, Svalbard. Mar Pollut Bull 77:367–374
Hao ZL, Wang F, Yang HZ (2013) Baseline values for heavy metals in soils on Ny-Alesund, Spitsbergen Island, Arctic: the extent of anthropogenic pollution. Adv Mater Res 779:1260–1265
Headley AD (1996) Heavy metal concentrations in peat profiles from the high Arctic. Sci Total Environ 177:105–111
Holt MS (2000) Sources of chemical contaminants and routes into the freshwater environment. Food Chem Toxicol 38:21–27
Jensen J (1999) Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment. Sci Total Environ 226:93–111
Jörundsdóttir HÓ, Jensen S, Hylland K, Holth TF, Gunnlaugsdóttir H, Svavarsson J, Ólafsdóttir A, El-Taliawy H, Rigét F, Strand J, Nyberg E, Bignert A, Hoydal KS, Halldórsson HP (2014) Pristine Arctic: background mapping of PAHs, PAH metabolites and inorganic trace elements in the North-Atlantic Arctic and sub-Arctic coastal environment. Sci Total Environ 493:719–728
Kadko D, Galfond B, Landing WM, Shelley RU (2016) Determining the pathways, fate, and flux of atmospherically derived trace elements in the arctic ocean/ice system. Mar Chem 182:38–50
Karasiński G, Posyniak M, Bloch M, Sobolewski P, Małarzewski Ł, Soroka J (2014) Lidar observations of volcanic dust over polish polar station at Hornsund after Eruptions of Eyjafjallajökull and Grímsvötn. Acta Geophys 62:316–339
Kolondra L (2003) Polish Polar Station and surrounding areas, Spitsbergen, orthophotomap 1:5000. Katowice (unpublished)
Kozak K, Polkowska Ż, Ruman M, Kozioł K, Namieśnik J (2013) Analytical studies on the environmental state of the Svalbard archipelago—critical source of information about anthropogenic global impact. Trends Anal Chem 50:107–126
Laing JR, Hopke PK, Hopke EF, Husain L, Dutkiewicz VA, Paatero J, Viisanen Y (2014) Long-term particle measurements in Finnish Arctic: Part I—chemical composition and trace metal solubility. Atmos Environ 88:275–284
Langmann B, Folch A, Hensch M, Matthias Y (2010) Volcanic ash over Europe during the eruption of Eyjafjallajökull on Iceland, April–May 2010. Atmos Environ 48:1–8
Larsen J, Appleby PG, Christensen GN, Berg T, Eide I (2010) Historical and geographical trends in sediment chronology from lakes and marine sites along the norwegian coast. Water Air Soil Pollut 206:237–250
Lawlor AJ, Tipping E (2003) Metals in bulk deposition and surface waters at two upland locations in northern England. Environ Pollut 12:153–167
Loska K, Wiechuła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Ma J, Hung H, Tian Ch, Kallenborn R (2011) Revolatilization of persistent organic pollutants in the Arctic induced by climate change. Nat Clim Change 1:255–260
Mann L, Long SE, Shuman ChA, Kell WR (2013) Determination of mercury content in a shallow firn core from Greenland by isotope dilution inductively coupled plasma mass spectrometry. Water Air Soil Pollut 163:19–32
Marszałek H, Wąsik M (2013) Some physico-chemical features of water in suprapermaforst zone in the Hornsund region (SW Spitsbergen) (Wybrane właściwości fizykochemiczne wód warstwy aktywnej wieloletnej zmarzliny w rejonie Hornsundu (SW Spitsbergen)). Biuletyn Państwowego Instytutu Geologicznego 456/2:497–403
Massart DL, Kaufman L (1983) The interpretation of analytical chemical data by the use of cluster analysis. J. Wiley, New York
Massart DL, Vandeginste BGM, Buydens LMC, De Jong S, Lewi PJ, Smeyers-Verbeke J (1998) Handbook of chemometrics and qualimetrics. Elsevier, Amsterdam
Mather TA, Pyle DM, Oppenheimer C (2003) Tropospheric volcanic aerosol. In: Robock A, Oppenheimer C (eds) Volcanism and the earth's atmosphere. Geophysical monograph series, vol 139. AGU, Washington, DC, pp 189–212
Matuła J, Pietryka M, Richter D, Wojtuń B (2007) Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area Spitsbergen. Polish Polar Res 4:283–315
Mearns AJ, Reish DJ, Oshida PS, Ginn T, Rempel-Hester MA, Arthur C, Rutherford N (2015) Effects of pollution on marine organisms. Water Environ Res 86:1869–1954
Melke J, Uziak S (2006) Heavy metals in soils and vascular plants of the Bellsund Area (Spitsbergen). Polish J Soil Sci 39:151–164
Nawrot AP, Migała K, Luks B, Pakszys P, Głowacki P (2016) Chemistry of snow cover and acidic snowfall during a season with a high level of air pollution on the Hans Glacier, Spitsbergen (in press), doi:http://dx.doi.org/10.1016/j.polar.2016.06.003
Niskavaara H, Reimann C, Chekushin V, Kashulina G (1997) Seasonal variability of total and easily leachable element contents in topsoils (0–5 cm) from eight catchments in the European Arctic (Finland, Norway and Russia. Environ Pollut 96:261–274
Nuttall M (2005) Encyclopedia of the Arctic, ISBN:1-57958-436-5, New York
Nystrand MI, Österholm P, Nyberg ME, Gustafsson JP (2012) Metal speciation in rivers affected by enhanced soil erosion and acidity. Appl Geochem 27:906–916
Parkhurst DL, Appelo CA (2000) User’s guide to PHREEQC (Version 2)—A computer program for speciation batch-reaction one-dimensional transport and inverse geochemical calculations. US Geological Survey 99-4259
Pecher K (1994) Hydrochemical analysis of spatial and temporal variations of solute composition in surface and subsurface waters of a high arctic catchment. Catena 214:305–327
Polkowska Ż, Cichała-Kamrowska K, Ruman M, Kozioł K, Krawczyk WE, Namieśnik J (2011) Organic pollution in surface waters from the Fuglebekken Basin in Svalbard, Norwegian Arctic. Sensors 11:8910–8929
Radziun K (1993) Observations on the Arctic charr. Salvelinus alpinus (L. 1758) from the Hornsund region (Vest Spitsbergen) 1985-1987. Acta Ichtyologica et Piscatoria 23:7–22
Reimann C, De Caritat D (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337:91–107
Robock A (2000) Volcanic eruptions and climate. Rev Geophys 38:191–219
Rose WI, Millard GA, Mather TA, Hunton DE, Anderson B, Oppenheimer C, Thornton BF, Gerlach TM, Viggiano AA, Kondo Y, Miller TM, Ballenthin LO (2006) Atmospheric chemistry of a 33–34 hour old volcanic cloud from Hekla Volcano (Iceland): Insights from direct sampling and the application of chemical box modeling. J Geophys Res 111, doi:10.1029/2005JD006872
Ruman M, Kozak K, Lehmann S, Kozioł K, Polkowska Ż (2012) Pollutants present in different components of the Svalbard Archipelago environment. Ecol Chem Eng S 19:571–584
Stachnik Ł, Majchrowska E, Yde JC, Nawrot AP, Cichała-Kamrowska K, Ignatiuk D, Piechota A (2016) Chemical denudation and the role of sulfide oxidation at Werenskioldbreen, Svalbard. J Hydrol 538:177–193. doi:10.1016/j.jhydrol.2016.03.059
Stock M, Ritter C, Herber A, Von Hoyningen-Huene W, Baibakov K, Gräser J, Orgis T, Treffeisen R, Zinoviev N, Makshtas A, Dethloff K (2008) Springtime Arctic aerosol: smoke versus haze, a case study for March 2008. Atmos Environ 52:48–55
Stunder B (1996) An assessment of the quality of forecast trajectories. J Appl Meteorol 35:1319–1331
Tepe N, Bau M (2014) Importance of nanoparticles and colloids from volcanic ash for riverine transport of trace elements to the ocean: Evidence from glacial-fed rivers after the 2010 eruption of Eyjafjallajökull Volcano, Iceland. Sci Total Environ 488:243–251
Vaisanen U, Misund A, Chekushin V (1998) Ecogeochemical investigation: stream water quality as an indicator of pollution in the border areas of Finland, Norway and Russia. Water Air Soil Pollut 104:205–219
Wagemann R, Lockhart WL, Welch H, Innes S (1995) Arctic marine mammals as integrators and indicators of mercury in the arctic. Water Air Soil Pollut 80:683–693
Wojtuń B, Samecka-Cymerman A, Kolon K, Kempers AJ, Skrzypek G (2013) Metals in some dominant vascular plants, mosses, lichens, algae, and the biological soil crust in various types of terrestrial tundra, SW Spitsbergen, Norway. Polar Biol 36:1799–1809
Zdanowicz C, Kruemmel E, Lean D, Poulain A, Kinnard C, Yumvihoze E, Chen J, Hintelmann H (2015) Pre-industrial and recent (1970-2010) atmospheric deposition of sulfate and mercury in snow on southern Baffin Island, Arctic Canada. Sci Total Environ 509:104–114
Acknowledgments
The authors would like to thank the staff of the Polish Polar Station Hornsund for the opportunity to carry out sampling and for their assistance with this work (special credit to the 33rd and 34th Polar Expedition of the Polish Academy of Sciences). Moreover, authors acknowledge Tomasz Wawrzyniak and the Hornsund Polish Polar Station staff for establishing and maintaining the meteorological station “Hornsund_01003” used in this investigation. We thank for the help in collecting samples and acknowledge the opportunity for direct observation of the study catchment as part of the Special Project 1173/IPY/2007 Higher Education of the 4th International Polar Year. This work was financed by the research Grant No. 2013/09/N/ST10/04191 (The National Science of Poland) and from the funds of the Leading National Research Centre (KNOW) received by the Centre for Polar Studies for the period 2014-2018. Furthermore, this work was also partially supported within statutory activities No 3841/E-41/S/2016 of the Ministry of Science and Higher Education of Poland. We gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model used in this publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M.Abbaspour
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kozak, K., Polkowska, Ż., Stachnik, Ł. et al. Arctic catchment as a sensitive indicator of the environmental changes: distribution and migration of metals (Svalbard). Int. J. Environ. Sci. Technol. 13, 2779–2796 (2016). https://doi.org/10.1007/s13762-016-1137-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1137-6