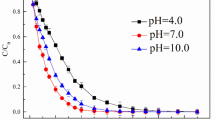
Abstract
Chlorate is one of the disinfection byproducts that are formed when chlorine/chlorine dioxide is used as a primary disinfectant. This study investigated the removal of chlorate by photochemical degradation using an advanced reduction process, which is a treatment method that combines a reducing agent with an activating method to generate reducing radicals. The effectiveness of combinations of reducing agents and three UV light sources having a peak output at 254, 365, and 312 nm were evaluated for chlorate removal. Dithionite irradiated by broad-band UVB lamp having the peak energy at 312 nm showed the highest chlorate removal. In pursuit of finding the optimum advanced reduction process conditions, the environmental process variables including pH, reducing agent dose, and light intensity were investigated. Dithionite/UV-B advanced reduction process was effective in weakly acidic conditions (pH < 5), and chlorate removal occurred in two steps. The first was an initial rapid decrease in chlorate concentration that occurred before initiating UV irradiation and was attributed to reaction with dithionite decomposition products. The second step was a slow decrease during UV irradiation that is caused by radicals produced by photolysis of the products of dithionite decomposition. The major product of chlorate destruction was chloride, with negligible amounts of chlorite produced.







Similar content being viewed by others
References
Aieta ME, Berg JD (1986) A review of chlorine dioxide in drinking water treatment. J Am Water Works Assoc 78:62–72
Alfredo K, Adams C, Eaton A, Roberson JA, Standford B (2014) The potential regulatory implications of chlorate. American Water Works Association, Washington DC
Amonette JE, Szecsody JE, Schaef HT, Templeton JC, Gorby YA, Fruchter JS (1994) Abiotic reduction of aquifer materials by dithionite: a promising in situ remediation technology. In: Baalman RW, Gee GW (eds) In situ remediation: scientific basis for current and future technologies proceedings of the 33rd hanford symposium on health and the environment, Richland, Washington, Pacific Northwest Laboratory
Anastasi EM, Wohlsen TD, Stratton HM, Katouli M (2013) Survival of Escherichia coli in two sewage treatment plants using UV irradiation and chlorination for disinfection. Water Res 47:6670–6679
Atkin EW, Hoff JC, Lippy EC (1982) Water outbreak control: which disinfectant? J Environ Helath Perspect 46:7–12
Awtrey AD, Connick RE (1951) The absorption spectra of I2, I3 −, I−, IO3 −, S4O5 2− and S2O3 2−. J Am Chem Soc 73:1842–1843
Burlamacchi L, Guarini G, Tiezzi E (1968) Mechanism of decomposition of sodium dithionite in aqueous solution. Trans Faraday Soc 65:496–502
Čermák V, Smutek M (1974) Mechanism of decomposition of dithionite in aqueous solutions. Collect Czechoslov Chem Commun 40:3241–3264
Connick RE, Tam TM, Von Deuster E (1982) The equilibrium constant for the dimerization of bisulfite ion to form S2O5 2−. Inorg Chem 21:103–107
Cotruvo J (2013) Contaminant of the month: chlorate. Water Technol. http://www.watertechonline.com/articles/166173-contaminant-of-the-month-chlorate. Accessed 1 April 2013
de Carvalho LM, Schwedt G (2001) Polarographic determination of dithionite and its decomposition products: kinetic aspect, stabilizers, and analytical application. Anal Chim Acta 436:293–300
de Carvalho LM, Schwedt G (2005) Sulfite speciation by capillary zone electrophoresis. Determination of dithionite and its decomposition products sulfite, sulfate and thiosulfate in commercial bleaching agents. J Chromatogr A 1099:185–190
Dunitz JD (1956) The structure of sodium dithionite and the nature of the dithionite ion. Acta Crystallogr A 9:579–586
Ershov BG, Kelm M, Janata E (2000) Pulse radiolysis studies of the reactions of e −aq and ·OH with ClO3 − ions in aqueous solution. Radiat Phys Chem 59:309–312
Fischer M, Warneck P (1996) Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution. J Phys Chem 100:15111–15117
Geoffroy N, Demopoulos GP (2009) Reductive precipitation of elemental selenium from selenious acidic solutions using sodium dithionite. Ind Eng Chem Res 48:10240–10246
Ginkel VCG, Plugge CM, Stroom CA (1995) Reduction of chlorate with various energy substrates and inocula under anaerobic conditions. Chemosphere 31:4057–4066
Golding RM (1960) Ultraviolet absorption studies of the bisulphite-pyrosulphite equilibrium. J Chem Soc 3711-3716
Gonce N, Vudrias E (1994) Removal of chlorite and chlorate ions from water using granular activated carbon. Water Res 28:1059–1069
Gordon G, Slootmakers B, Tachiyashiki S, Wood DW (1990) Minimizing chlorite ion and chlorate ion in water treated with chlorine dioxide. J Am Water Works Assoc 82:160–165
Hayon E, Treinin A, Wilf J (1972) Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems. The SO2 −, SO3 −, SO4 − and SO5 − radicals. J Am Chem Soc 94:47–57
Holman DA, Bennett DW (1994) A multicomponent kinetics study of the anaerobic decomposition of aqueous sodium dithionite. J Phys Chem 98:13300–13307
Horváth AK, Nagypál I (2006) Kinetics and mechanism of the oxidation of sulfite by chlorine dioxide in a slightly acidic medium. J Phys Chem A 110:4753–4758
Jiang C, Li H, Lin C (2009) Effects of activated sludge on the degradation of chlorate in soils under varying environmental conditions. J Hazard Mater 162:1053–1058
Jung B, Nicola R, Batchelor B, Abdel-Wahab A (2014) Effect of low- and medium-pressure Hg UV irradiation on bromate removal in advanced reduction process. Chemosphere 117:663–672
Kroom AGM, Van Ginkel CG (2004) Biological reduction of chlorate in a gas-lift reactor using hydrogen as an energy source. J Environ Qual 33:2026–2029
Lambeth DO, Palmer G (1973) The kinetics and mechanism of electron transfer proteins and other compounds of biological interest by dithionite. J Biol Chem 248:6095–6103
Li H, Zhu X, Ni J (2011) Comparison of electrochemical method with ozonation, chlorination and monochloramination in drinking water disinfection. Electrochim Acta 56:9789–9796
Lindholm CA (1999) Handbook for pulp and paper tehcnologies vol 5. Chapter 11. Bleaching. TAPPI Press
Liu X, Yoon S, Batchelor B, Abdel-Wahab A (2013) Degradation of vinyl chloride (VC) by the sulfite/UV advanced reduction process(ARP): effect of process variables and a kinetic model. Sci Total Environ 454–455:578–583
Liu X, Vellanki BP, Batchelor B, Abdel-Wahab A (2014) Degradation of 1,2-dichloroethane with advanced reduction processes (ARPs): effects of process variables and mechanisms. Chem Eng J 237:300–307
Lynn S, Rinker RG, Corcoran WH (1964) The monomerization rate of dithionite ion in aqueous solution. J Phys Chem 68:2363
Makarov SV (2001) Recent trends in chemistry of sulfur-containing reductants. Usp Khim 70:996–1007
Malmqvist Å, Welander T (1994) Biological removal of chlorate from bleaching plant effluent. Water Sci Technol 29:365–372
Nixon AC, Krauskopf KB (1932) The rate of reaction between chlorate and sulfur dioxide in acid solution. J Am Chem Soc 54:4606–4608
Rinker RG (1959) I. Studies in the chemistry of sodium dithionite. II. A preliminary study of the catalyzed addition of hydrogen chloride to vinyl chloride in a stirred reactor. Ph.D, California Institute of Technology
Rinker RG, Gordon TP, Corcoran WH (1964) Electron spin resonance studies of sodium dithionite and sodium formaldehyde sulfoxylate. Inorg Chem 3:1467–1469
Spencer MS (1967) Chemistry of sodium dithionite. Trans Faraday Soc 2510–2515
Stanford BD, Pisarenko AN, Snyder SA, Gordon G (2011) Perchlorate, bromate, and chlorate in hypochlorite solutions: guidelines for utilities. J Am Water Works Assoc 103:71–83
Sun W, Sierra- Alvarez R, Milner L, Field J (2010) Anaerobic oxidation of arsenite linked to chlorate reduction. J Appl Environ Microbiol 76:6804–6811
Szirovicza L (2009) The kinetics of the chlorate-bisulfite and chlorate-sulfite/bisulfite reaction systems. React Kinet Catal Lett 96:311–317
USEPA (1999) Alternative disinfectants and oxidants guidance manual. Environmental Protection Agency, Office of Water, Washington DC
Vellanki BP, Batchelor B (2013) Perchlorate reduction by the sulfite/ultraviolet light advanced reduction process. J Hazard Mater 262:348–356
Vellanki BP, Batchelor B, Abdel-Wahab A (2013) Advanced reduction processes: a new class of treatment processes. Environ Eng Sci 30:264–271
WHO (2004) Guidelines for drinking-water quality. WHO guidelines, vol 1. World Health Organization, Geneva
Yoon S, Han DS, Liu X, Batchelor B, Abdel-Wahab A (2014) Degradation of 1,2-dichloroethane using advanced reduction processes. J Environ Chem Eng 2:731–737
Acknowledgments
This study was made possible by a grant from the Qatar National Research Fund under its National Priorities Research Program award number NPRP 4-1174-2-458. The paper’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the Qatar National Research Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, B., Sivasubramanian, R., Batchelor, B. et al. Chlorate reduction by dithionite/UV advanced reduction process. Int. J. Environ. Sci. Technol. 14, 123–134 (2017). https://doi.org/10.1007/s13762-016-1132-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1132-y