Abstract
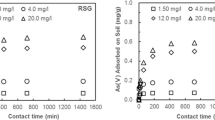
Previous studies in our laboratory have demonstrated that drinking-water treatment residuals are effective sorbents of arsenic V. However, the effect of soil solution chemistry on arsenic V sorption by drinking-water treatment residuals-amended soils remains to be explored. The current study uses a batch incubation experimental set up to evaluate the effect of soil solution pH, competing ligands, and complexing metal on arsenic V sorption by a sandy soil (Immokalee series) amended with two rates (25 and 50 g kg−1) of aluminum and iron-based drinking-water treatment residuals. Experiments were conducted at three initial arsenic loads (125, 1,875, 3,750 mg kg−1) and a constant solid: solution ratio of 200 g L−1. An optimum equilibration time of 8 days, obtained from kinetic studies, was utilized for sorption experiments with both aluminum and iron drinking-water treatment residual-amended soil. Presence of phosphate decreased arsenic V sorption by both aluminum and iron drinking-water treatment residual amended soils, with a strong dependence on pH, drinking-water treatment residual types, drinking-water treatment residual application rates, and phosphate concentrations. Addition of sulfate had no effect on arsenic V sorption by aluminum or iron drinking-water treatment residual-amended soil. A complementing effect of calcium on arsenic V sorption was observed at higher pH. Results elucidating the effect of soil solution chemistry on the arsenic V sorption will be helpful in calibrating drinking-water treatment residual as a sorbent for remediation of arsenic-contaminated soils.






Similar content being viewed by others
References
Ben-Dor E, Banin A (1989) Determination of organic matter content in arid-zone soils using a simple “loss-on-ignition” method. Commun Soil Sci Plant Anal 20:1675–1695
Elliott HA, Dempsey BA (1991) Agronomic effects of land application of water treatment sludges. J. AWWA 83:126–131
Elliott HA, O’Connor GA, Brinton S (2002) Phosphorus leaching from biosolids-amended sandy soils. J Environ Qual 31:681–689
Goh K, Lim T (2004) Geochemistry of inorganic arsenic and selenium in a tropical soil: effect of reaction time, pH and competitive anions on arsenic and selenium adsorption. Chemosphere 55:849–859
Goldberg S (2002) Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci Soc Am J 66:413–421
Hanlon EA, Gonzalez JS, Bartos JM (1997a) Soil pH (1:2v/v). IFAS extension soil testing laboratory (ESTL) and analytical research laboratory (ARL) chemical procedures and training manual. Fl. Coop. Ext. Ser. Cir. 812, University of Florida, Gainesville, FL, 15
Hanlon EA, Gonzalez JS, Bartos JM (1997b) Electrical conductivity. IFAS extension soil testing laboratory (ESTL) and analytical research laboratory (ARL) chemical procedures and training manual. Fl. Coop. Ext. Ser. Cir. 812, University of Florida, Gainesville, FL, 24
Jain A, Loeppert RH (2000) Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J Environ Qual 29:1422–1430
Livesey NT, Huang PM (1981) Adsorption of Arsenate by soils and its relation to selected chemical properties and anions. Soil Sci 131:88–94
Loeppert RH, Inskeep WP (1996) Iron. In: Method of soil analysis. Part 3: chemical methods. SSSA Book Series, pp 639–664
Makris KC, Harris WG, O’Connor GA, Obreza TA (2004) Phosphorus immobilization in micropores of drinking-water treatment residuals: implications for long-term stability. Environ Sci Technol 38:6590–6596
Makris KC, Harris WG, O’Connor GA, Obreza TA, Elliott HA (2005) Physicochemical properties related to long-term phosphorus retention by drinking water treatment residuals. Environ Sci Technol 39:4280–4289
Makris KC, Sarkar D, Datta R (2006) Evaluating a drinking-water waste by-product as a novel sorbent for arsenic. Chemosphere 64:730–741
Makris KC, Sarkar D, Parsons JG, Datta R, Gardea-Torresdey JL (2007) Surface arsenic speciation of a drinking-water treatment residual using X-ray absorption spectroscopy. J Colloid Interface Sci 311:544–550
Manful GA, Verloo M, Spiegeleer FG (1989) Arsenate sorption by soils in relation to pH and selected anions. Pedologie 39:55–68
Manning BA, Goldberg S (1996) Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Sci Soc Am J 60:121–131
Nagar R, Sarkar D, Makris KC, Datta R, Sylvia VL (2009) Bioavailability and bioaccessibility of arsenic in soil amended with drinking-water treatment residuals. Arch Environ Contam Toxicol 59:755–766
Nagar R, Sarkar D, Makris KC, Datta R (2010) Effect of solution chemistry on arsenic sorption by Al- and Fe-based drinking-water treatment residuals. Chemosphere 78:1028–1035
O’Reilly SE, Strawn DG, Sparks DL (2001) Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci Soc Am J 65:67–77
Raven KP, Jain A, Loeppert RH (1998) Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ Sci Technol 32:344–349
Sall J, Creighton L, Lehman A (2005) JMP start statistics, 3rd edn. SAS Institute, 631 Cary, NC
Sarkar D, Makris KC, Vandanapu V, Datta R (2007) Arsenic immobilization in soils amended with drinking-water treatment residuals. Environ Pollut 146:414–419
Sparks DL (1999) Kinetics and mechanism of chemical reactions at the soil mineral/water interface. In: Soil physical chemistry. 2nd edn. CEC Press, Boca Raton, pp 135–191
Williams LE, Barnett MO, Kramer TA, Melville JG (2003) Adsorption and transport of arsenic(V) in experimental subsurface systems. J Environ Qual 32:841–850
Yang Y, Zhao YQ, Babatunde AO, Wang L, Ren YX, Han Y (2006) Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Separ Purif Technol 51:193–206
Acknowledgments
The authors gratefully acknowledge NIH-SCORE, SALSI, and USEPA-STAR programs for funding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagar, R., Sarkar, D., Makris, K.C. et al. Inorganic arsenic sorption by drinking-water treatment residual-amended sandy soil: effect of soil solution chemistry. Int. J. Environ. Sci. Technol. 10, 1–10 (2013). https://doi.org/10.1007/s13762-012-0106-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0106-y