Abstract
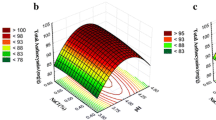
Wine prepared from the tropical fruit bael (Aegle marmelos L.), known for its medicinal properties, is a novel beverage rich in antioxidants having enough credibility for projecting it as a medicinal wine. In the present study, bael, which is abundantly available in the region, was selected for wine production and its alcohol content was estimated using gas chromatography analysis. The conditions for the fermentation were optimised using response surface methodology. The quantitative effects of temperature, pH and duration of fermentation were investigated on fermentation of ethanol from bael using wine yeast Saccharomyces cerevisiae (NCIM 3315) as the starter culture. The optimum conditions for a 8.13% yield of ethanol were found to be temperature of 34℃, pH of 4.5 and duration of fermentation of 48 h. Biochemical analyses were conducted to study the proximate attributes of the pulp and wine with their respective changes due to fermentation. The raw bael pulp had total soluble solids 22.31°Brix, pH 5.33, total phenolics 1.94 g/100 ml and carotenoids 32.98 µg/g dw; the wine had total soluble solids 3.3°Brix, pH 4.54, total phenolics 0.96 g/100 ml and carotenoids 29.90 µg/g dw. The bael pulp and wine had 2,2-diphenyl-1- picrylhydrazyl (DPPH)-scavenging activity of 70.99% and 49.03% respectively. The Hunter colour values of the bael pulp and wine were also evaluated and the results are presented. In this study, heavy metals like Cu (0.219 mg/l), Pb (0.190 mg/l), Zn (0.002 mg/l) and Fe (3.296 mg/l) were found in the wine. The structural feasibility of bael as an effective substrate for fermentation was also confirmed by scanning electron microscopy studies.
Similar content being viewed by others
References
Duarte WF, Dias DR, de Melo Pereira GV, Gervasio IM, Schwan RF (2009) Indigenous and inoculated yeast fermentation of gabiroba (Campomanesia pubescens) pulp for fruit wine production. J Ind Microbiol Biot 36:557–569
Panda SK, Sahu UC, Behera SK, Ray RC (2014) Bio-processing of bael [Aegle marmelos L.] fruits into wine with antioxidants. Food Biosci 5:34–41
Di Stefano R, Guidoni S (1989) The analysis of total phenols in must and wines. Vignevivni 1:47–52
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Suvimol C, Pranee A (2008) Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. Int Food Res J 15:45–63
Acetoy M, Abollinoz O, Bruzzonit MC, Mentastiz E, Sarzaniniz C, Malandrinoz M (2002) Determination of metals in wine with atomic spectroscopy (flame-AAS, GF-AAS and ICP-AES): a review. Food Addit Contam 19:126–133
Torija MJ, Beltran G, Novo M, Poblet M, Manuel J, Mas GA, Roze N (2003) Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int J Food Microbiol 85:127–136
Pino JA, Queris O (2011) Analysis of volatile compounds of mango wine. Food Chem 125:1141–1146
Jagtap UB, Waghmare SR, Lokhande VH, Suprasanna P, Bapat VA (2011) Preparation and evaluation of antioxidant capacity of Jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Ind Crop Prod 34:1595–1601
Varakumar S, Kumar YS, Reddy OVS (2011) Carotenoid composition of Mango (Magnifera indica L.) wine and its antioxidant activity. J Food Biochem 35:1538–1547
Mohanty S, Ray P, Swain MR, Ray RC (2006) Fermentation of Cashew (Anacardium occidentale L.) “Apple” into wine. J Food Process Pres 30:314–322
Chowdhury P, Ray RC (2007) Fermentation of Jamun (Syzgium cumini L.) fruits to form red wine. ASEAN Food J 14:15–23
Kumar DP, Tiwari A, Bhat R (2004) Effect of pH on the stability and structure of yeast hexokinase A acedic amino acid residues in the cleft region are critical for the opening and the closing of the structure. J Biol Chem 279:32093–32099
Sahu UC, Panda SK, Mohapatra UB, Ray RC (2012) Preparation and evaluation of wine from tendu (Diospyros melanoxylon L.) fruits with antioxidants. Int J Food Ferment Technol 2(2):167–178
Gorinstein S, Weisz M, Zemser M, Tilis K, Stiller A, Flam I, Gat Y (1993) Spectroscopic analysis of polyphenols in white wines. J Ferment Bioeng 75:115–120
Czyzowska A, Pogorzelski E (2002) Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part I. Total polyphenols and phenolic acids. Eur Food Res Technol 214:148–154
González Sanjosé ML, Izcara E, Pérez-Magariño S, Revilla I (1998) Modification of red wine color using pectolytic enzymes. In Charbonnier F, Delacotte JM, Rolando (eds) Polyphenol Communications 98, XIXth International Conference on Polyphenols, Lille
Nuengchamnong N, Ingkaninan K (2010) On-line HPLC–MS–DPPH assay for the analysis of phenolic antioxidant compounds in fruit wine: Antidesma thwaitesianum Muell. Food Chem 118:147–152
Heinonen IM, Lehtonen PJ, Hopia AI (1998) Antioxidant activity of berry and fruit wines and liquors. J Agric Food Chem 46:25–31
Orescanin V, Katunar A, Kutle A, Valkovic V (2003) Heavy metals in soil, grape, and wine. J Trace Microprobe Tech 21:171–180
Scollary GR (1997) Metals in wine: contamination, spoilage and toxicity. Analusis 25:26–30
Lara R, Cerutti S, Salonia JA, Olsina RA, Martinez LD (2005) Trace element determination of Argentine wines using ETAAS and USN-ICP-OES. Food Chem Toxicol 43:293–297
Reddy LV, Reddy LP, Wee YJ, Reddy OVS (2011) Production and characterization of wine with sugarcane piece immobilized yeast biocatalyst. Food Bioprocess Technol 4:142–148
Jouenne T, Baonato H, Mignot L, Junter GA (1992) Cell immobilization in composite agar layer microporous membrane structures: growth kinetics of gel-entrapped cultures and cell leakage limitation by microporous membrane. Appl Microbiol Biotechnol 38:478–481
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, K., Saha, J., Raychaudhuri, U. et al. Optimization of bioprocessing parameters using response surface methodology for bael (Aegle marmelos L.) wine with the analysis of antioxidant potential, colour and heavy metal concentration. Nutrafoods 14, 39–49 (2015). https://doi.org/10.1007/s13749-014-0064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13749-014-0064-8