Abstract
An analysis of adult population fluctuation of Anastrepha ludens (Loew) was performed in southern Tamaulipas, Mexico from 2008 to 2011. The aim was to analyze population dynamics of A. ludens and its relationships with climatic factors in the citrus region of Llera, Tamaulipas, Mexico. Population densities were weekly examined to identify variation through the year and study period. Four periods were identified according to population size, amplitude, host availability and season of the year. The correlation between population density vs. rainfall and temperature (average, minimum and maximum) was determined by linear and multiple regression analyses. Simple linear regression analysis showed that population density with minimum temperature and rainfall was the most consistent correlation, whereas in multiple regression analysis, rainfall and maximum temperature showed more consistency. A seasonal association between the availability of commercial host, climatic variation, and population peaks of A. ludens was determined. This study may have practical implications for the design of specific control strategies, monitoring, and infestation prevention based on different phases of the pest through the year. This strategy, along with the area-wide approach implemented by the Plant Protection Service may lead to an optimization of material, financial and human resources.
Similar content being viewed by others
Introduction
The Mexican fruit fly, Anastrepha ludens (Loew), is one of the most important pests of citrus in Mexico, Belize, Guatemala, and the lower Rio Grande Valley in Texas (Aluja et al 1996, Thomas & Loera-Gallardo 1998). Major damage is caused when the larvae feed on the pulp of the ripe fruit, leading to deterioration in quality and premature fruit drop, accounting for great economic losses to producers. About 37% of fruit injuries provoked by infestations of A. ludens can occur in Mexico (Lopéz-Arroyo & Loera-Gallardo 2009), although higher damages have been observed in Tamaulipas, especially in grapefruit.
In the southern region of the state, in contrast to municipalities located in the central portion, control activities of the National Campaign Against Fruit Flies (NCAFF) are generally carried out at local scale (orchard). Ground bait sprays are basically the control method used, which have little impact on pest populations because of the continuous reinfestation of neighboring groves (Aluja et al 2012). This situation is particularly so in the case of Llera, Tamaulipas, aggravated by the geographic location of this citrus area between the foothills of the Sierra Madre Oriental (SMO) and Sierra de Tamaulipas.
An additional characteristic of the region is the scarce presence of the wild host “yellow chapote,” Casimiroa greggi (Plummer et al 1941, Thomas 2003) at the foothills of the SMO or close to groves as occurs at the central region. This could suggest a population flow toward the orchards or vice versa (Quintero et al 2009). The tropical condition of the region promotes common presence of host in backyards such as mango (Mangifera indica) and sour orange (Citrus aurantium).
The fluctuation of adult populations of Anastrepha spp. has been documented in several studies in tropical areas of Mexico (Celedonio-Hurtado et al 1995, Aluja et al 1996, Martínez-Morales et al 2003, Tucuch-Cahuich et al 2008, Aluja et al 2012, Ordano et al 2013) and elsewhere in the American continent (Houston 1981, Nguyen et al 1992, Hedström 1993, Ronchi-Teles & Da Silva 2005, Montes et al 2012). However, in general, these studies have reported discrepant results on the association between population dynamics and climatic factors. This suggests local behavior because of different host availability, along with different environmental and ecological conditions on each study area.
In northeastern Mexico, A. ludens is considered a native pest (Plummer et al 1941, Thomas 2003, Quintero et al 2009) exhibiting high citrus infestations during the ripening period. Fruit damage is detected on sweet citrus such as Valencia orange, grapefruit, and mandarins, probably associated with seasonality and climatic factors. Tephritidae populations of Ceratitis capitata Wiedemann and Bactrocera dorsalis (Hendel) have been linked to climatic variables, ripening and host availability (Chen & Ye 2007, Appiah et al 2009).
At local scale (orchard), dispersion studies on sterile flies of A. ludens have reported weak correlations between catches with the maximum temperature and rainfall in northern Tamaulipas (Thomas & Loera-Gallardo 1998). In the state of Nuevo León, Mexico, Thomas (2003, 2012) studied population fluctuation of A. ludens associated with its wild host Casimiroa greggi in the Sierra Madre Oriental, and a significant correlation with rainfall was determined.
This work aimed to analyze the population dynamics of A. ludens from 2008 to 2011 and to evaluate its relationship with climatic variables in the citrus area of Llera, Tamaulipas, Mexico.
Material and Methods
Study area
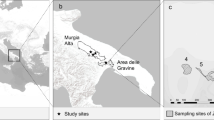
The citrus area at the municipality of Llera is located in southern Tamaulipas between 23°18′ and 23°07′N and 99°03′ and 98°44′W at km 65 southern Victoria city, Tamaulipas capital, and 120 km away from the citrus area in the central portion of the state. This region has about 4300 ha of citrus groves; the main citrus variety is Valencia orange, followed by grapefruits and mandarins (SAGARPA 2013). The altitude varies from 150 to 300 m above sea level and the west side of the region borders with the Sierra Madre Oriental. The geographic orientation of the study area is from north to south along Guayalejo riverbed (Fig 1).
Sampling
Data obtained from 137 McPhail traps deployed by NCAFF during the period 2008 to 2011 were used to determine adult population fluctuation of A. ludens. These data were provided by the Comité Estatal de Sanidad Vegetal de Tamaulipas (CESAVETAM). McPhail traps were baited with four tablets (20 g) of torula yeast (Trampol 300®, Grupo PAUSA S.A. de C.V) diluted in 250–300 mL of water and hung in a tree between 3 and 4 m above the ground. Traps were serviced every 7 days and distributed at a distance of 120 to 150 m between them, trying to achieve a uniform network. Host availability was the main criterion for placing the traps, although in large citrus areas different sweet varieties are observed in an intercalate way inside the orchards or near them.
Weekly trap captures were transformed to flies per trap per day (FTD) values by using the formula total number of flies / (number of traps × days of trap exposure). This parameter is expressed with four digits after zero (IAEA 2013). Thus, variability in trap number or capture periods was standardized (Aluja et al 2012).
Weather data
Records of four climatic variables from 2008 to 2011 were used as follows: weekly average of mean, minimum, maximum temperature (Celsius), and rainfall (millimeters). Data were obtained from a weather station operated by the Comisión Nacional del Agua (CNA) at Ejido Emilio Carranza (23°15′18″N, 99°49′60′′W) in the central part of the citrus area.
Data analysis
A descriptive statistical analysis of the weekly FTD per year was performed. The citrus variety, climatic oscillations, and weekly FTD were taken into account to determine infestation periods. This is because of the importance of population variations in short periods of time and its implications in control activities and monitoring.
Following Aluja et al (2012), monthly FTD data were considered to work (i.e., averaged over 4 weeks) coupled with data of climatic variables that were calculated as monthly averages, as well. Because of high variability of monthly FTD data, these values were transformed to log10 that enabled to stabilize the variance and use the data for further analysis.
By using FTD transformed data and a statistical significance of p ≤ 0.05, FTD was considered as a response variable and climatic variables data as explanatory potential factors in a simple linear regression. Coefficient of determination (r 2) was utilized to judge the best fit of the data. Also, the contribution of an explanatory variables combination was determined by using a multiple regression model. The forward stepwise method was used to individually add or delete the independent variables from the model at each step of the regression, until the best fit model was obtained. These analyses were carried out with STATISTICA 6.1 program (StatSoft 2004).
Results and Discussion
Population fluctuation
Weekly FTD values pointed higher values of the mean over the median and positive skewness as well (Table 1). This suggests heterogeneity of FTD values (captures) and non-normally distributed data, which is very common in insect population (García 2006). Population peaks were consistently observed in certain periods with a variation in the FTD level. Therefore, the population dynamics of A. ludens was classified into fourth periods through the year based on FTD level, production season, and climatic variations (Fig 2 and Table 2).
The highest population densities were observed in the period January–April, particularly in March to early spring, which is related to the higher citrus production (Valencia orange). The Valencia orange represents about 78% of the citrus crops in Tamaulipas (SAGARPA 2013); thus, agroecosystem complexity gives advantages to the reproductive potential of A. ludens, as well as other Tephritidae such as Ceratitis capitata, due to the wide access to host availability, shelter, and food (Aluja 1994, Appiah et al 2009, Aluja & Rull 2009).
According to SAGARPA (2013), about 60% of citrus growing in Tamaulipas belongs to the ejido system. This is an important socioeconomic factor, because there are generally economic necessities of the growers in the first 2 months of the year, so they sell their product at that time. In this way, fruit availability in March–April is likely to be lower than in January and February. However, FTD showed the lowest value at the beginning of the year, whereas the highest in March, respectively (Fig 2 and Table 2). This latter occurred when the Valencia orange had almost achieved the optimum level of ripeness.
In an integrated pest management, large captures of insect pest in traps usually have been associated with a high population density (Bearup et al 2015), but it could also be related to a greater search for food, fly physiological condition, age, population structure, and environmental conditions (Hendrichs et al 1991, Baker & Chan 1991, Thomas et al 2001, Díaz-Fleischer et al 2009). There are many aspects in A. ludens biology, which are not taken into account in the control strategies of the NCAFF in Tamaulipas. In Llera, for example, suppression activities are focused on the population peaks by applying ground bait sprays, which have little impact on the pest populations. We assumed that several generations are involved in the infestation on Valencia orange; thus, demographic parameters as age structure would be determinants for a strategic planning (Carey 1982).
Another condition is the presence of ripe grapefruit of the previous year, which represents a continuous focus of infestation. The commercialization of grapefruit in the last months of the year is very important to suppress the population increase and damage of A. ludens next year, because of its preference for this citrus (Baker et al 1944, Robacker & Fraser 2002, Thomas 2003, Birke et al 2006). Although the grapefruit area is smaller than the Valencia orange, grapefruit trees are commonly found throughout the citrus area in the orchards or backyards.
A second period was considered from May to mid-August and is characterized by a drastic reduction of the Valencia orange production, along with an increase of temperature by the advent of the summer season. By this time, the harvest is almost over, and only some growers from the private sector maintain the fruit in the tree (Valencia orange) trying to obtain better prices. Although several phytosanitary measures are performed in these orchards in order to preserve the fruit, harvest residues represent a source of local infestations.
A wide range in the FTD values was detected, from 0.0000 in 2010 to 0.5251 in 2008 (Table 2). It is noteworthy that the size of the population peaks in this period was determined by the level of pest populations in January–April (Fig 2), but these populations declined over a short period of time due to the scarcity of commercial hosts. Pest outbreaks after maximum host availability have been reported for Anastrepha spp. by Aluja (1994) and Celedonio-Hurtado et al (1995). Population growth after the main harvest may be attributed to the search of food for flies, which promotes greater catches in traps (Baker & Chan 1991).
On the other hand, at this time, the ripeness of yellow chapote (Table 3) could explain the high captures in traps. However, the scarcity of the wild host in southern Tamaulipas is an advantage with respect to the citrus areas located at central Tamaulipas, which are in many cases bordered by yellow chapote populations. Therefore, in the citrus region of Llera, there is less probability of movement of fruit flies from the wild to the nearest citrus areas. Southernmost distribution of yellow chapote in Tamaulipas has not been established; it has been observed growing some 70 km south of Ciudad Victoria (Plummer et al 1941).
A third period based on the relative absence of citrus production and the presence of high temperatures (up to 47°C) was determined and comprises only 5 weeks (August–September). This phase preceded the initiation of ripening of early varieties, mainly grapefruits. Zero values were observed in several cases that suggest null prevalence (NOM-023-FITO-1995) of A. ludens populations in the orchards. High temperatures along with the host availability play an important role in the population size of B. dorsalis and Ceratitis capitata elsewhere in the world (Chen & Ye 2007, Appiah et al 2009).
The scarcity of captures may be because of the distances among the traps (120–150 m), and not because of effective control actions (Celedonio-Hurtado et al 1995). In studies at local scale (orchard) on adult populations of Anastrepha suspensa (Loew) and Ceratitis capitata, it was determined that effective trapping ranges were between 30 and 40 m by using different attractants (Epsky et al 2010, Kendra et al 2010).
Another aspect is the role of alternating host that serves as shelter and breeding sites in the absence of high availability of commercial hosts (Aluja 1994, Aluja & Rull 2009). For multivoltine fruit flies, the absence of fructification of its main host may be solved by exploiting alternative hosts (Aluja & Mangan 2008), as is the case with sour orange or “cucha” present all year in backyards (Table 3). Likewise, A. ludens seems to have developed physiological and behavioral strategies at different scenarios of host availability (Aluja et al (2011). Nonetheless, other factors such as lack of marketing of the product and harvest residues in the orchards may also contribute to the sustainability of local populations of the pest.
Lastly, a fourth period from September to December was considered and included the ripening phase of grapefruit, mandarin, and early oranges (Table 3). FTD range was lower than the first and the second peaks and fluctuated between 0.0042 and 0.2044 (Table 2). This outbreak was consistent and extended from the end of September until December, where the highest FTD level may continue until early next year. In addition, a temperature decline caused by the arrival of winter was observed (Fig 3). It is suggested that this is the most important second peak after January–April, because of the beginning of the citrus commercial season, where the grapefruit is a representative variety in the period. It occupies about 80 ha in Llera (SAGARPA 2013) and has intercalated presence with Valencia orange in mixed orchards (Aluja 1994), or backyards, which provides food and host for A. ludens in the entire region at this time of year.
The adult population of A. ludens was lower than in the other periods; however, in tropical areas, the number of flies detected in grapefruit orchards can be almost equal to the number of flies captured in orange orchards (Houston 1981). A high population of larvae was detected in the area throughout the study, mainly in October–November. About 60% of fruit damage in a grapefruit orchard caused by A. ludens has been documented by the NCAFF in the centre of Tamaulipas. The above coincides with Thomas (2003) that pointed out the importance of oviposition on grapefruit in October and November, which leads to the emergence of adults in January–February. In this case, an earlier emergence of adults occurred since November–December and had a tendency to continue until the end of the Valencia orange season as seen in 2008–2009 (Fig 2).
It should be noted that larvae and egg stages of A. ludens comprises about 68% of a total population (Liedo et al 1993). Therefore, pest population dynamics in this period seems to be an important basis for designing specific regional strategies in an area-wide approach aimed at preventing high populations in Valencia orange, with a low potential impact on non-target insect population (Thomas 2012, Ordano et al 2013).
FTD and climatic variables
The presence of commercial hosts (oranges, grapefruits, and mandarins) modulates A. ludens population density in the citrus areas of Llera. Additionally, changes in climatic conditions, especially changes in temperature and rainfall, came along with the FTD variation during the study.
Annual accumulated rainfall ranged from 72 mm in 2011 to 151 mm in 2008. The average temperature showed values from 11°C in winter to 32°C in summer. Regarding maximum temperatures, these data fluctuated between 16 and 47°C (Fig 3). Thus, differences were observed between average (5°C) and maximum temperature (15°C) in winter and summer, respectively. Thomas (2012) reported for the Sierra Madre Oriental in the state of Nuevo León, Mexico, lower ranks between average and maximum temperature (5–8°C), as well as higher cumulative annual rainfall (129–481 mm).
The FTD of 2009 did not show any significant correlation with any climatic variable. FTD variations during the study period may be the factor that influences correlations with the independent variables. This is most evident in the FTD of 2011, which showed values of dispersion measures (variance and standard deviation) lower than those in other years (Table 1) of the study. Consequently, less variation in the FTD could have contributed to the higher coefficients of determination (r 2) in 2011. Similarly, the scatterplots did not reveal any pattern involving non-parametric tests.
A significant relationship (p < 0.05) of the FTD transformed data with rainfall and temperature per year (except 2009) was obtained. Minimum temperature and rainfall were the most consistent correlations with the FTD throughout the study. The most representative correlations for each variable and FTD were observed in 2011 (r 2 > 0.60), in which FTD with average temperature was the highest value (r 2 = 0.64, Table 4).
At local level (orchard), weak relationships between captures of A. ludens sterile adults, maximum temperature, and rainfall (Thomas & Loera-Gallardo 1998) have been reported in northern Tamaulipas. In tropical areas, high temperatures, rainfall, and abundance of host have been associated significantly with high populations of Anastrepha spp. (Celedonio-Hurtado et al 1995, Montes et al 2012). Contrary, to the north of Mexico, tropical areas show an average temperature relatively constant through the year (Celedonio-Hurtado et al 1995, Ronchi-Teles & Da Silva 2005).
Minimum temperature recorded in winter, as low as 0°C, did not affect A. ludens populations drastically. The lowest populations at freezing temperatures were observed in 2010 (Fig 2), but these temperatures were not maintained for a long time. Survival of Tephritidae populations at low temperatures has been reported for Ceratitis capitata and A. ludens on citrus areas and at upper sites in the Sierra Madre Oriental, respectively (Israely et al 1997, Martínez-Ferrer et al 2010, Thomas 2012). On average, December and January were the coldest months (Fig 3); it seems to be a critical point for A. ludens, but grapefruit and early orange infestations played an important role to maintain pest populations (Table 2). In addition, the formation of large compact mosaics of citrus growing provides diverse habitats at different spatial scales (Aluja & Rull 2009), which include backyard alternative hosts as Citrus aurantium (sour orange) in urban areas.
Temperature influence has been discarded as an important factor on the population dynamics of Anastrepha spp., in southern areas (Celedonio-Hurtado et al 1995, Ronchi-Teles & Da Silva 2005). However, in northern Mexico, the role of temperature seems to be more important on the number of generations of A. ludens on its native host in the Sierra Madre Oriental by accumulation of degree-days (Leyva-Vázquez 1988, Thomas 2003, 2012). Likewise, in the citrus areas, a seasonal association between the host availability and temperature changes was determined.
The highest rainfall occurred from the end of April through September, so there is no match between the main population peaks of the insect and wide host availability (January–March and October–December, Fig 4). This has been reported also for Anastrepha spp., from tropical areas of the American continent (Hedström 1993, Ronchi-Teles & Da Silva 2005, Montes et al 2012). However, in nature, rainfall is closely related to the fruiting stage of yellow chapote, the wild host of A. ludens in the Sierra Madre Oriental (Plummer et al 1941, Thomas 2003, 2012).
The highest correlation value of rainfall with FTD was obtained in 2010 (r 2 = 0.62). This contribution may be considered significant taking into account that the host availability is the regulatory factor in population size (Hedström 1993, Aluja 1994, Celedonio-Hurtado et al 1995, Ronchi-Teles & Da Silva 2005). In this study, high rainfall probably has an important role in sustaining A. ludens low populations during the scarcity of commercial hosts in the period May–August.
Moreover, correlation values may be low compared with those obtained by Thomas (2012) between precipitation and adult captures (r 2 = 0.926) in the Sierra Madre Oriental. However, irrigation activities represent a variable in the citrus areas that may replace the effect of precipitation at local scale. This moisture can vary from a wilting point to field capacity during certain periods, exposing pupae of A. ludens to different ranges of soil moisture. The immature stages of A. ludens have shown a high adaptive capacity to these conditions (Montoya et al 2008).
The correlation of rainfall and maximum temperature with FTD in the multiple regression models was the best fit of the data (Table 5). As in the simple linear regression, relationships of FTD with any climatic variable were not determined in 2009. Correlation of FTD with rainfall was the most consistent in the 3 years. Rainfall has been associated with A. ludens populations in a delayed way influenced by the global climate phenomena in a citrus area (Aluja et al 2012).
The summer season has two characteristics, high temperature and rainfall, which are not linked to high infestations of A. ludens, but rather related to low populations in the citrus areas. This finding and the lack of host availability provide agro-ecological conditions to suppress pest populations, in contrast to wild population in the Sierra Madre Oriental, where two or three generations are reported (Thomas 2003, 2012).
Our results show that, the size and amplitude of the peak populations of A. ludens were modulated by the availability of the commercial host throughout the year. Fruit fly population affecting the main commercial season was concentrated in a period of 7 months (October–April). Furthermore, FTD relationships with temperature and rainfall were determined by host availability, along with seasonal changes. Considering that control actions of the NCAFF are usually more reactive rather than preventive, this study may contribute to a more appropriate management of this pest considering its population dynamics and the related factors. This would have important implications for control and monitoring activities at regional level, and an optimization of financial and human resources.
References
Aluja M (1994) Bionomics and management of Anastrepha. Ann Rev Entomol 39:155–178
Aluja M, Celedonio–Hurtado H, Liedo P, Cabrera M, Castillo F, Guillen J, Rios E (1996) Seasonal population fluctuations and ecological implications for management of Anastrepha fruit flies (Diptera: Tephritidae) in commercial mango orchards in southern Mexico. J Econ Entomol 89:654–667
Aluja M, Mangan R (2008) Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological and regulatory considerations. Ann Rev Entomol 53:473–502
Aluja M, Rull J (2009) Managing pestiferous fruit flies (Diptera: Tephritidae) through environmental manipulation. In: Aluja M, Leskey TC, Vincent C (eds) Biorational tree fruit pest management. CAB International, Wallingford, pp 171–213
Aluja M, Birke A, Guillén L, Díaz-Fleischer F, Nestel D (2011) Coping with an unpredictable and stressful environment: the life history and metabolic response to variable food and host availability in a polyphagous tephritid fly. J Insect Physiol 57:1592–1601
Aluja M, Ordano M, Guillen L, Rull J (2012) Understanding long-term fruit fly (Diptera: Tephritidae) population dynamics: implications for area-wide management. J Econ Entomol 105:823–836
Appiah FE, Afreh-Nuamah K, Obeng-Ofori D (2009) Abundance and distribution of the Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae), in Late Valencia citrus orchards in Ghana. Int J Trop Insect Sci 29:11–16
Baker PS, Chan AST (1991) Appetitive dispersal of sterile fruit flies: aspects of the methodology and analysis of trapping studies. J Appl Entomol 112:263–273
Baker W, Stone E, Plummer CC, McPhail M (1944) A review of studies on the Mexican fruit fly and related Mexican species. USDA Miscellaneous Publication No 531, Washington D.C., p 152
Bearup D, Petrovskaya N, Petrovskii S (2015) Some analytical and numerical approaches to understanding trap counts resulting from pest insect immigration. Math Biosci 263:143–160
Birke A, Aluja M, Greany P, Bigurra E, Pérez-Staples D, McDonald R (2006) Long aculeus and behavior of Anastrepha ludens render gibberellic acid ineffective as an agent to reduce “ruby red” grapefruit susceptibility to the attack of this pestiferous fruit fly in commercial groves. J Econ Entomol 99:1184–1193
Carey JR (1982) Demography and population dynamics of the Mediterranean frui fly. Ecol Model 16:125–150
Celedonio-Hurtado H, Aluja M, Liedo P (1995) Adult population fluctuation of Anastrepha species (Diptera: Tephritidae) in tropical orchards habitats of Chiapas, México. Environ Entomol 24:861–869
Chen P, Ye H (2007) Population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) and analysis of factors influencing populations in Baoshanba, Yunnan, China. Entomol Sci 10:141–147
de Agricultura S, Ganadería DR, Pesca y Alimentación (2013) Padrón de productores de cítricos georeferenciados 2010–2012 Tamaulipas. Delegación Estatal. Estadística Agropecuaria, Cd. Victoria, p 48
Díaz-Fleischer F, Arredondo J, Flores S, Montoya P, Aluja M (2009) There is no magic trap fruit fly trap: multiple biological factors influence the response of adult Anastrepha ludens and Anastrepha oblicua (Diptera: Tephritidae) individuals to Multilure traps baited with Biolure or Nulure. J Econ Entomol 102:86–94
Epsky ND, Espinoza HR, Kendra PE, Abernathy R, Midgarden D, Heath RR (2010) Effective sampling range of a synthetic protein-based attractant for Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol 103:1886–1895
García FJM (2006) Analysis of the spatio-temporal distribution of Helicoverpa armigera Hb. in a tomato field using stochastic approach. Biosyst Eng 93:253–259
Hedström I (1993) Population dynamics and host relationships of Neotropical flies (Diptera: Tephritidae) in seasonal and non-seasonal environments. Int J Manag 39:400–410
Hendrichs J, Katsoyannos BI, Papaj DR, Procopy RJ (1991) Sex differences in movement between natural feeding and mating sites and tradeoffs between food consumption, mating success and predator evasion in Mediterranean fruit flies (Diptera: Tephritidae). Oecologia 86:223–231
Houston WWK (1981) Fluctuations in numbers and the significance of the sex ratio of the Mexican fruit fly Anastrepha ludens caught in McPhail traps. Entomol Exp Appl 30:140–150
International Atomic Energy Agency (2013) Guidelines for the Use of Mathematics in Operational Area-Wide Integrated Pest Management Programmes Using the Sterile Insect Technique with a Special Focus on Tepehritid Fruit Flies. Insect Pest Control Section, International Atomic Energy Agency, Vienna, p 102
Israely N, Yuval B, Kitron U, Nestel D (1997) Population fluctuations of adult Mediterranean fruit flies (Diptera: Tephritidae) in a Mediterranean heterogeneous agricultural region. Environ Entomol 26:1263–1269
Kendra EP, Epsky DN, Heath RR (2010) Effective sampling range of food-based attractants for female Anastrepha suspensa (Diptera: Tephritidae). J Econ Entomol 103:533–540
Leyva-Vázquez JL (1988) Temperatura umbral y unidades calor requeridas por los estados inmaduros de Anastrepha ludens (Loew) (Diptera: Tephritidae). Folia Entomol Mex 74:189–196
Liedo P, Carey JR, Celedonio H, Guillen J (1993) Demography of Anastrepha fruit flies: a case of study of three species of economic importance. In: Aluja M, Liedo P (eds) Fruit flies biology and management. Springer, New York, pp 67–72
Lopéz-Arroyo JI, Loera-Gallardo J (2009) Manejo Integrado de Insectos y Acaros Plaga de los Cítricos. In: Rocha-Peña MA, Padrón-Chávez JE (eds) El Cultivo de los Cítricos en el Estado de Nuevo León, vol 1, Libro Científico No. Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. CIRNE. Campo Experimental General Terán, México, pp 260–323
Martínez-Ferrer MT, Navarro C, Campos JM, Marzal C, Fibla JM, Bargues L, Garcia-Marí F (2010) Seasonal and annual trends in field populations of Mediterranean fruit fly, Ceratitis capitata, in Mediterranean citrus groves: comparison of two geographic areas in eastern Spain Span. J Agric Res 8:757–765
Martínez-Morales A, Alia-Tejacal I, Hernández-Hernández UL (2003) Fluctuación poblacional de moscas de la fruta, género Anastrepha (Diptera: Tephritidae), en una huerta de zapote mamey en Jalpa de Méndez, Tabasco, México. Cent Agríc 4:54–59
Montes SMNM, Raga A, De Souza-Filho MF (2012) Occurrence of fruit flies (Diptera: Tephritidae) in a mixed mango orchard in the city of Presidente Prudente, SP, Brazil. Rev Colomb Entomol 38:231–237
Montoya P, Flores S, Toledo J (2008) Effect of rainfall and soil moisture on survival of adults and immature stages of Anastrepha ludens and A. oblicua (Diptera: Tephritidae) under semi-field conditions. Fla Entomol 91:643–650
Nguyen R, Poucher C, Brazzel JR (1992) Seasonal occurrence of Anastrepha suspensa (Diptera: Tephritidae) in Indian River County, Florida, 1984–1987. J Econ Entomol 85:813–820
NOM-023-FITO-1995. Norma Oficial Mexicana por la que se establece la Campaña Contra Moscas de la Fruta. Publicada en el Diario Oficial de la Federación el 11 febrero 1999. México, p 18
Ordano M, Guillén L, Rull J, Lasa R, Aluja M (2013) Temporal dynamics of diversity in a tropical fruit fly (Tephritidae) ensemble and their implications on pest management and biodiversity conservation. Biodivers Conserv 22:1557–1575
Plummer CC, McPhail M, Monk WJ (1941) The yellow chapote, a native host of the Mexican fruit fly. USDA. Technique Bulletin 775, Washington D.C., p 12
Quintero VP, Arroyo JIL, Gallardo JL, Rull J, Robles ER, Mondaca EC, Delgado SH, Perez NM, Schuneman MA (2009) Genetic differences between Anastrepha ludens (Loew) populations stemming from a native and an exotic host in NE Mexico. Agric Técnica Méx 35:323–331
Robacker DC, Fraser I (2002) Do Mexican fruit flies (Diptera: Tephritidae) prefer grapefruit to yellow chapote, a native host? Fla Entomol 85:481–487
Ronchi-Teles B, Da Silva NM (2005) Flutuação populacional de espécies de Anastrepha Schiner (Diptera: Tephritidae) na Região de Manaus, AM. Neotrop Entomol 34:733–741
StatSoft Inc (2004) STATISTICA (data analysis software system) version 6. www.statsoft.com
Thomas DB (2003) Reproductive phenology of the Mexican fruit fly, Anastrepha ludens (Loew) (Diptera: Tephritidae) in the Sierra Madre Oriental, northern Mexico. Neotrop Entomol 32:385–397
Thomas DB (2012) Mexican fruit fly (Diptera: Tephritidae) and the phenology of its native host plant yellow chapote (Rutaceae) in Mexico. J Entomol Sci 47:1–16
Thomas D, Loera-Gallardo J (1998) Dispersal and longevity of mass-released, sterilized Mexican fruit flies (Diptera: Tephritidae). Environ Entomol 27:1045–1052
Thomas DB, Holler TC, Heath RR, Salinas EJ, Moses AL (2001) Trap lure combinations for surveillance of Anastrepha fruit flies (Diptera: Tephritidae). Fla Entomol 84:344–351
Tucuch-Cahuich F, Chi Que G, Orona-Castro F (2008) Dinámica poblacional de adultos de la mosca mexicana de la fruta Anastrepha sp. (Díptera: Tephritidae) en Campeche, México. Agric Técnica Méx 34:341–347
Acknowledgments
We thank Luis Guardiola and Guillermo Gerónimo from NCAFF for their technical assistance, and to the Consejo Nacional de Ciencia y Tecnología (CONACyT) for financial support to V. Vanoye to carry out doctoral studies. We also thank the authorities of the Comité Estatal de Sanidad Vegetal de Tamaulipas by grant permission to use trapping database in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Wesley AC Godoy – ESALQ/USP
Rights and permissions
About this article
Cite this article
Vanoye-Eligio, V., Barrientos-Lozano, L., Pérez-Castañeda, R. et al. Population Dynamics of Anastrepha ludens (Loew) (Diptera: Tephritidae) on Citrus Areas in Southern Tamaulipas, Mexico. Neotrop Entomol 44, 565–573 (2015). https://doi.org/10.1007/s13744-015-0328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-015-0328-z