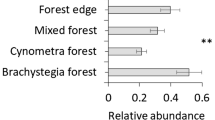
Abstract
The determinants of diversity are a central issue in ecology, particularly in Andean forests that are known to be a major diversity hotspot for several taxa. We examined the effect of abiotic (elevation and precipitation) and biotic (flowering plant diversity) factors considered to be decisive causal factors of diversity patterns on anthophyllous insect communities on mountain forest. Sampling was carried out in 100-m transects at eight elevational levels and during a period of 8 months. All flowering plants in the understory and their flowering visitors were recorded. Species richness and diversity were estimated for each elevation and month. Diversity of flowering plants, elevation, and precipitation were used as independent variables in multiple regressions against insect diversity. The evaluated abiotic and biotic factors had contrasting effects on insect diversity: a significant decrease on insect diversity occurred at high elevation and dry months (i.e., threshold effect), while it showed a positive relationship with flowering plant diversity through time (i.e., linear effect), but not along elevation. Rapid turnover of species of both interacting guilds was observed every 100-m altitude and month. Local insect communities were also divided functionally depending on the plant family they visit. These results indicate that each insect community is distinctive among elevations and months and that diversity of flowering plants, precipitation, and elevation influence their structure and composition. Thus, conservation strategies should involve protection of forest cover at the whole elevation gradient, in order to preserve common and exclusive components of diversity and consequently, the mosaic of plant–pollinator interactions.





Similar content being viewed by others
References
Alves-Silva E, Barönio GJ, Torezan-Silingardi HM, Del-Claro K (2013) Foraging behavior of Brachygastra lecheguana (Hymenoptera: Vespidae) on Banisteriopsis malifolia (Malpighiaceae): extrafloral nectar consumption and herbivore predation in a tending ant system. Entomol Sci 16:162–169
Arnott SE, Vanni MJ (1993) Zooplankton assemblages in fishless bog lakes: influence of biotic and abiotic factors. Ecology 74:2361–2380
Arroyo MTK, Primack R, Armesto JJ (1982) Community studies in pollination ecology in the high temperate Andes of Central Chile. I. Pollination mechanisms and altitudinal variation. Am J Bot 69:82–97
Bachman S, Baker WJ, Brummit N, Dransfield J, Moat J (2004) Elevational gradients, area and tropical island diversity: an example from the palms of New Guinea. Ecography 27:299–310
Basilio AM, Medan D, Torretta JP, Bartoloni NJ (2006) A year-long plant–pollinator network. Austral Ecol 31:975–983
Basset Y (2001) Invertebrates in the canopy of tropical rain forests—how much do we know? Plant Ecol 153:87–107
Brehm G, Süßenbach D, Fiedler K (2003) Unique elevational patterns of geometrid moths in an Andean montane rainforest. Ecography 26:456–466
Brehm G, Pitkin LM, Hilt N, Fiedler K (2005) Montane Andean rain forests are a global diversity hotspot of geometrid moths. J Biogeogr 32:1621–1627
Callejas R, Idarraga A (2011) Flora de Antioquia. Catálogo de las plantas vasculares, vol I, Introducción. D’Vinni, Bogotá
Cardona-Duque J, Gómez-Murillo L, Franz NM (2011) Phylogenetic reassessment of Cyclanthura, a Neotropical genus of Acalyptini associated with arum and cyclanth inflorescences (Coleoptera: Curculionidae: Curculioninae). Annual Meeting of the Entomological Society of America, Reno NV, XI-14-2011
Carranza-Quiceno JA, Estévez-Varón JV (2008) Ecología de la polinización de Bromeliaceae en el dosel de los bosques neotropicales de montaña. Boletín Científico Centro de Museos. Museo de Historia Natural 12:38–47
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Austral J Ecol 18:117–143
Colwell RK (2009) EstimateS: statistical estimation of species richness and shared species from samples, version 8.2 (http://purl.oclc.org/estimates)
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Phil Trans R Soc B 345:101–118
Condon MA, Scheffer SA, Lewis ML, Swensen SM (2008) Hidden Neotropical diversity: greater than the sum of its parts. Science 320:928–931
Cuesta F, Peralvo M, Valarezo N (2009) Los bosques montanos de los Andes Tropicales: una evaluación regional de su estado de conservación y de su vulnerabilidad a efectos del cambio climático. Imprenta Mariscal, Quito
Devoto M, Medan D, Montaldo NH (2005) Patterns of interaction between plants and pollinators along an environmental gradient. Oikos 109:461–472
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Dyer LA, Singer MS, Lill JT, Stireman JO, Gentry GL, Marquis RJ, Ricklefs RE, Greeney HF, Wagner DL, Morais HC, Diniz IR, Kursar TA, Coley PD (2007) Host specificity of Lepidoptera in tropical and temperate forests. Nature 448:696
Escobar F, Lobo JM, Halffter G (2005) Altitudinal variation of dung beetle (Scarabaeidae: Scarabaeinae) assemblages in the Colombian Andes. Glob Ecol Biogeogr 14:327–337
Faegri K, Van Der Pijl L (1979) The principles of pollination ecology, 3rd edn. Pergamon, London
Fisher BL (1998) Ant diversity along an elevational gradient in the Réserve Spéciale d’Anjanaharibe-Sud and on the western Masoala Peninsula, Madagascar. Fieldiana Zool 90:39–67
Guisande-Gonzáles C, Vaamonde A, Barreiro A (2011) Tratamiento de datos con R, Statistica y SPSS. FER Fotocomposición, España
Hammer Ø (2011) PAleontological STatistics PAST. Version 2.12. Natural History Museum. University of Oslo, Norway
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513
Hoffman F (2005) Biodiversity and pollination. Flowering plants and flower-visiting insects in agricultural and semi-natural landscapes. PhD. Thesis, University of Groningen. Groningen, Netherlands, p 224
Kearns CA, Inouye DW (1997) Pollinators, flowering plants, and conservation biology. Bioscience 47:297–307
Kelly CK, Southwood TRE (1999) Species richness and resource availability: a phylogenetic analysis of insects associated with trees. Proc Natl Acad Sci U S A 96:8013–8016
Kessler M (2000) Altitudinal zonation of Andean cryptogam communities. J Biogeogr 21:275–282
Kessler M, Kromer T (2000) Patterns and ecological correlates of pollination modes among bromeliad communities of Andean forest in Bolivia. Plant Biol 2:659–669
Korner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574
Krömer T, Kessler M, Herzog SK (2006) Distribution and flowering ecology of bromeliads along two climatically contrasting elevational transects in the Bolivian Andes. Biotropica 38:183–195
Lawton JH, MacGarvin M, Heads PA (1987) Effects of altitude on the abundance and species richness of insect herbivores on bracken. J Anim Ecol 56:147–160
Ledesma-Castañeda EA (2011) Plan de manejo Reserva Natural La Mesenia-Paramillo. BSc Thesis. Servicio Nacional de Aprendizaje SENA, Caldas, Antioquia, Colombia
Lobo JM, Halffter G (2000) Biogeographical and ecological factors affecting the altitudinal variation of mountainous communities of coprophagous beetles (Coleoptera, Scarabaeoidea): a comparative study. Ann Entomol Soc Am 93:115–126
Lomolino MV (2001) Elevation gradients of species–density: historical and prospective views. Glob Ecol Biogeogr 10:3–13
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Malo JE, Baonza J (2002) Are there predictable clines in plant–pollinator interactions along altitudinal gradients? The example of Cytisus scoparius (L.) link in the Sierra de Guadarrama (Central Spain). Divers Distrib 8:365–371
Manly BFJ (1991) Randomization and Monte Carlo methods in biology. Chapman and Hall, London
Novotny V, Drozd P, Miller SE, Kulfan M, Janda M, Basset Y, Weiblen GD (2006) Why are there so many species of herbivorous insects in tropical rainforests? Science 313:1115–1118
Petanidou T, Kallimanis AS, Tzanopoulos JS, Gardeli SP, Pantis JD (2008) Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecol Lett 11:564–575
Pinheiro F, Diniz IR, Coelho D, Bandeira MPS (2002) Seasonal pattern of insect abundance in the Brazilian Cerrado. Austral Ecol 27:132–136
Power ME, Stout RJ, Cushing CE, Harper PP, Hauer FR, Matthew WJ, Moyle PB, Statzner B, Wais de Badgen IR (1988) Biotic and abiotic controls in river and stream communities. J N Am Benthol 7:456–479
Pyrcz TW, Wojtusiak J (2002) The vertical distribution of pronophiline butterflies (Nymphalidae, Satyrinae) along an elevational transect in Monte Zerpa (Cordillera de Mérida, Venezuela) with remarks on their diversity and parapatric distribution. Glob Ecol Biogeogr 11:211–221
Rahbeck C (1995) The elevational gradient of species richness: a uniform pattern? Ecography 18:200–205
Rico-Gray V, Díaz-Castelazo C, Ramírez-Hernández A, Guimarães PR Jr, Holland JN (2012) Abiotic factors shape temporal variation in the structure of an ant–plant network. Arthropod Plant Interact 6:289–295
Suárez YR, Junior MP, Catella AC (2004) Factors regulating diversity and abundance of fish communities in Pantanal lagoons, Brazil. Fish Manag Ecol 11:45–50
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Vásquez JA, Givnish TJ (1998) Altitudinal gradients in tropical forest composition, structure and diversity in the Sierra de Manatlán. J Ecol 86:999–1020
Vilela AA, Torezan-Silingardi HM, Del-Claro K (2014) Conditional outcomes in ant-plant-herbivore interactions influenced by sequential flowering. Flora. doi:10.1016/j.flora.2014.04.004
Wolda H (1987) Altitude, habitat and tropical insect diversity. Biol J Linn Soc 30:313–323
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26:1101–1108
Acknowledgments
Thanks to The Hummingbird Conservancy and Gustavo Suárez for the logistic support. We are greatly indebted to Martha Wolff (Colección Entomológica de Antioquia) for the assistance with identification of insects, Felipe Cardona and Alvaro Idarraga for the assistance with identification of plants (Herbario Universidad de Antioquia), and Liliana Ramírez and local assistants from Family Rendón-Agudelo for the field job. This work was supported by grant Comité para el Desarrollo de la Investigación CODI-Universidad de Antioquia CPT-0915.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Kleber Del Claro – UFU
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Supplementary Material S1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Cuartas-Hernández, S.E., Gómez-Murillo, L. Effect of Biotic and Abiotic Factors on Diversity Patterns of Anthophyllous Insect Communities in a Tropical Mountain Forest. Neotrop Entomol 44, 214–223 (2015). https://doi.org/10.1007/s13744-014-0265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-014-0265-2