Abstract
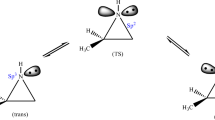
In the present study, the experimentally observed regioselectivity in pyrimido[4,5-b][1,4]benzothiazine synthesis has been modeled using density functional theory method at M06/6-311++G** level. Also the tautomerism of all four tautomers of 6-ethyl-2-thio(1H) pyrimidin-(3H)-4-one as an important intermediate through the reaction path in pyrimido[4,5-b][1,4]benzothiazine synthesis has been investigated extensively in the gas and solution phase. The stability order of tautomers was found as A > D > B > C, in gas and chloroform solvent; however, changing to more polar solvents this order was changed as A > B > D > C using polarized continuum model. In the next step, we focused on another significant feature of this synthesis which has major role in the observed regioselectivity. Two proposed reaction paths with two different transition states that it seems the mode of its intermolecular cyclization has a special role in regioselectivity of the final product have been considered. Comparison of our calculated NMR and IR spectrum with those already reported for some pyrimido[4,5-b][1,4]benzothiazines demonstrates a reliable agreement. Moreover, all obtained results in the gas and solution phase confirm that the synthesis of above-mentioned compound is thermodynamically more favorable than the possible regioisomeric product.








Similar content being viewed by others
References
B. Ramesh, C.M. Bhalgat, Eur. J. Med. Chem. 46, 1882 (2011)
R. Sawant, V. Sarode, Iran. J. Pharm. Res. 10, 733 (2011)
V.L. Gein, V.V. Mishunin, E.P. Tsyplyakova, O.V. Vinokurova, M.I. Vakhrin, Pharm. Chem. J. 43, 652 (2009)
G.R. Revankar, T.S. Rao, K. Ramasamy, D.F. Smee, Nucleosides Nucleotides 14, 671 (1995)
F.D. Smee, H.A. Alaghmandan, K. Ramasamy, G.R. Revankar, Antiviral Res. 26, 203 (1995)
D.G. Kini, J.D. Anderson, Y.S. Sanghvi, A.F. Lewis, D.F. Smee, G.R. Revankar, K. Robins, K. Ronald, H.B. Cottam, J. Med. Chem. 34, 3006 (1991)
E.S.A.M. Badawey, S.M. Rida, A.A. Huzza, H.T.Y. Fahmy, Y.M. Gohar, Eur. J. Med. Chem. 28, 91 (1993)
P.G. Higgins, G.I. Barrow, D.A.J. Tyrrel, N.J.C. Snell, K. Jones, W.B. Jolley, Antiviral Chem. 2, 61 (1991)
D.F. Smee, J.H. Huffman, A.C. Gessman, J.W. Huggins, R.W. Sidwell, Antiviral Res. 15, 229 (1991)
T.S. Rao, G.R. Revankar, R.S. Vinayak, R.K. Robins, J. Heterocycl. Chem. 28, 1779 (1991)
V.E. Steele, C.A. Holmes, E.T. Hawak, L. Kopelovich, R.A. Lubet, J.A. Crowell, C.C. Sigman, G.F. Kellof, Cancer Epidemiol. Biomark. Prev. 8, 467 (1999)
J.M. Schwab, C.N. Serhan, Curr. Opin. Pharmacol. 6, 414 (2006)
M. Okita, D.C. Gaudette, G.B. Mills, B.J. Holub, Int. J. Cancer 71, 31 (1997)
M. Croset, N. Brossard, A. Polette, M. Lagarde, Biochem. J. 345, 61 (2000)
A.R. Brash, J. Biol. Chem. 274, 23679 (1999)
T. Schewe, J. Biol. Chem. 383, 365 (2002)
U. Kelavkar, W. Glasgow, T.E. Eling, Curr. Urol. Rep. 3, 207 (2002)
U.P. Kelavkar, C. Cohen, H. Kamitani, T.E. Eling, K.F. Badr, Carcinogenesis 21, 1777 (2000)
J. Zhu, I. Kilty, H. Granger, E. Gamble, Y.S. Qiu, K. Hattotuwa, W. Elston, W.L. Liu, A. Liva, R.A. Pauwels, J.C. Kis, V. De Rose, N. Barnes, M. Yeadon, S. Jenkinson, P.K. Jeffery, Am. J. Respir. Cell Mol. Biol. 27, 1044 (2002)
H.G. Johnson, M.L. McNee, F.F. Sun, Am. Rev. Respir. Dis. 131, 917 (1985)
A. Brown, A. Henderson, S. Jenkinson, I. Kilty, S. Liu, S. Monaghan, T. Wood, M. Yeadon, Drugs Future 27, C55 (2002)
L. Zhao, C.D. Funk, Trends Cardiovasc. Med. 14, 191 (2004)
J.A. Cornicell, B.K. Trivedi, Curr. Pharm. Des. 5, 11 (1999)
M.M. Heravi, T. Alishiri, Adv. Heterocycl. Chem. 113, 1 (2014)
M.M. Heravi, B. Talaei, Adv. Heterocycl. Chem. 113, 143 (2014)
M.M. Heravi, S. Khaghaninejad, M. Mostofi, Adv. Heterocycl. Chem. 112, 1 (2014)
M.M. Heravi, S. Khaghaninejad, N. Nazari, Adv. Heterocycl. Chem. 112, 183 (2014)
M.M. Heravi, B. Talaei, Adv. Heterocycl. Chem. 114, 147 (2015)
M.M. Heravi, V.F. Vavsari, Adv. Heterocycl. Chem. 114, 77 (2015)
S. Khaghaninejad, M.M. Heravi, Adv. Heterocycl. Chem. 45, 38 (2014)
M.M. Heravi, V. Zadsirjan, Adv. Heterocycl. Chem. 117, 261 (2015)
M.M. Heravi, G. Rajabzadeh, F.F. Bamoharram, J. Mol. Catal. A: Chem. 256, 238 (2006)
M.M. Heravi, S. Sadjadi, H.A. Oskooie, R.H. Shoar, F.F. Bamoharram, Tetrahedron Lett. 50, 662 (2009)
M.M. Heravi, K. Aghapoor, M.A. Nooshabadi, M.M. Mojtahedi, Monatsh. Chem. 128, 1143 (1997)
I. Tinoco, C. Bustamante, J. Mol. Biol. 293, 271 (1999)
B. Andersh, K.N. Kilby, M.E. Turnis, D.L. Murphy, J. Chem. Educ. 85, 1 (2008)
S. Dadiboyena, E.J. Valente, A.T. Hamme, Tetrahedron Lett. 55, 2208 (2014)
R.M. Balabin, J. Chem. Phys. 131, 154307 (2009)
A. Steinbach, A.J. Scheidig, C.D. Klein, J. Med. Chem. 51, 5143 (2008)
K. Vazdar, M. Vazdar, Tetrahedron Lett. 56, 6908 (2015)
X. Zhang, Int. J. Quantum Chem. 115, 1658 (2015)
F.S. Hashemi, F. Forooghian, M.M. Heravi, THEOCHEM 770, 123 (2006)
H.R. Memarian, F. Rezaie, H. Sabzyan, M. Ranjbar, J. Iran. Chem. Soc. 11, 1265 (2014)
T. Hosseinnejad, M.M. Heravi, R. Firouzi, J. Mole. Model. 19, 951 (2013)
R. Mirsafaei, M.M. Heravi, S. Ahmadi, M.H. Moslemin, T. Hosseinnejad, A. Chemical. 402, 100 (2015)
T. Hosseinnejad, B. Fattahi, M.M. Heravi, J. Mol. Model. 21, 1 (2015)
R.G. Parr, W. Yang, Density-Functional Theory of Atoms and Molecules (Oxford University Press, Oxford, 1989)
D.G. Truhlar, Y. Zhao, Theor. Chem. Acc. 120, 215 (2008)
K. Fukui, Acc. Chem. Res. 14, 363 (1981)
V. Barone, M. Cossi, J. Tomasi, J. Comp. Chem. 19, 404 (1998)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A. Montgomery, R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G.A. Petersson, P.Y. Ayala, Q. Cui, K. Morokuma, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J. Cioslowski, J.V. Ortiz, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, C.V. Ghonzalez, M. Challacombe, P.M.W. Gill, B.G. Johnson, W. Chen, M. Wong, J.L. Andres, M. Head-Gordon, E.S. Replogle, J.A. Pople, Gaussian 2003 (Revision-B) (Gaussian Inc, Pittsburgh, 2003)
M. Navarrete, C. Rangel, J.C. Corchado, J. Phys. Chem. A 109, 4777 (2005)
H.Y. Zhang, H.F. Ji, THEOCHEM 667, 167 (2003)
M. Bakavoli, M. Nikpour, M. Rahimizadeh, M.R. Saberi, H. Sadeghian, Bioorg. Med. Chem. 15, 2120 (2007)
K. Wolinski, J.F. Hilton, P.J. Pulay, J. Am. Chem. Soc. 112, 8251 (1990)
Acknowledgements
The authors are thankful to Alzahra University Research Council. MMH is thankful also to Iran National Scientific Foundation (INSF) for partial financial support, allocated for his individual research chair.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ghiasi, M., Aghawerdi, M. & Heravi, M.M. QM study on regioselectivity in the synthesis of pyrimido[4,5-b][1,4]benzothiazines: kinetic and thermodynamic point of view. J IRAN CHEM SOC 14, 743–754 (2017). https://doi.org/10.1007/s13738-016-1014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-1014-8


