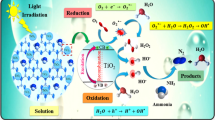
Abstract
The reduction of Ni(II) ion, originated from nitrate or sulfate salts, was investigated based on photo-generated electrons in UV-irradiated TiO2 aqueous suspensions. Design of experiments, modeling, and process optimization were performed using central composite design of response surface methodology. Influence of pH, temperature, and nickel concentration was investigated based on percentage of reduction efficiency (RE). Under operating conditions of pH = 9.3, T = 40 °C, [Ni(II)]o = 5 mg L−1, [TiO2] = 100 mg L−1 and after 90 min treatments, 64.8 and 76.1 % RE were achieved for nitrate and sulfate counter-anions, respectively. The higher efficiency obtained with sulfate anion was attributed to the more ionic strength and its interaction with titania nanoparticles. Rate of Ni(II) ions reduction, originated from both of the nickel salts, obeys pseudo-first-order kinetic model. As a relevant criterion, the electrical energy consumption and other criteria were evaluated and were compared with other previously reported processes.




Similar content being viewed by others
References
K.M. Joshi, B.N. Patil, D.S. Shirsath, V.S. Shrivastava, Adv. Appl. Sci. Res. 2, 445–454 (2011)
S. Veli, B. Alytiz, J. Hazard. Mater. 149, 226–233 (2007)
M. Caicedo, J.J. Jacobs, A. Reddy, N.J. Hallab, J. Biomed. Mater. Res. 86, 905–913 (2008)
M.A. Barakat, Y.T. Chen, C.P. Huang, Appl. Catal. B Environ. 53, 13–20 (2004)
D. Chen, A.K. Ray, Chem. Eng. Sci. 56, 1561–1570 (2001)
M. Shirzad Siboni, M.T. Samadi, J.K. Yang, S.M. Lee, Desalin. Water Treat. 40, 77–83 (2012)
E. Malkoc, Y. Nuhoglu, J. Hazard. Mater. 135, 328–336 (2006)
S. Lacour, J.C. Bollinger, B. Serpaud, D. Chantron, R. Arcos, Anal. Chem. Acta 428, 121–132 (2001)
W. Wu, J.J. Peng, J. Taiwan Inst. Chem. Eng. 42, 498–505 (2011)
D.S. Bhatkhande, V.G. Pangarkar, A.A. Beenackers, J. Chem. Technol. Biotechnol. 77, 102–116 (2001)
J. Saien, A. Azizi, A.R. Soleymani, Sep. Purif. Technol. 134, 187–195 (2014)
I.K. Konstantinou, T.A. Albanis, Appl. Catal. B Environ. 42, 319–335 (2003)
R.S. Thakur, R. Chaudhary, C. Singh, Desalin. Water Treat. 56, 1335–1363 (2015)
M.N. Chong, B. Jin, C.W.K. Chow, C. Saint, Water Res. 44, 2997–3027 (2010)
N. Bao, G. Wu, J. Niu, Q. Zhang, S. He, J. Wang, J. Alloys Compd. 599, 40–48 (2014)
T. Lavanya, K. Satheesh, M. Dutta, N.V. Jaya, N. Fukata, J. Alloys Compd. 615, 643–650 (2014)
M. Abdullah, G.K.C. Low, R.W. Matthews, J. Phys. Chem. 94, 6820–6825 (1990)
H. Zhu, M. Zhang, Z. Xia, G.K.C. Low, Water Res. 29, 2681–2688 (1995)
C.R. Chenthamarakshan, H. Yang, Y. Ming, K. Rajeshwar, J. Electroanal. Chem. 494, 79–86 (2000)
J. Saien, A. Azizi, Process Saf. Environ. Prot. 95, 114–125 (2015)
J. Saien, H. Delavari, A.R. Solymani, J. Hazard. Mater. 177, 1031–1039 (2010)
K. Kabra, R. Chaudhary, R.L. Sawhney, Environ. Prog. 27, 487–495 (2008)
M. Shirzad Siboni, M.T. Samadi b, J.K. Yang, S.M. Lee, Environ. Technol. 32, 1573–1579 (2011)
J. DeZuane, Handbook of Drinking Water Quality: Standards and Controls (Van Nostrand Reinhold, New York, 1990)
L.S. Clesceri, A.E. Greenberg, A.D. Eaton, Standard Methods for the Examination of Water and Wastewater (American Public Health Association, Washington, DC, 1998)
D.C. Montgomery, Design and Analysis of Experiments, 5th edn. (Wiley, New York, 2001)
R.A. Burns, J.C. Crittenden, D.W. Hand, V.H. Selzer, L.L. Sutter, S.M. Salman, J. Environ. Eng. 125, 77–85 (1999)
J.M. Meichtry, C.C. Justin, G. Custoa, M.I. Litter, Appl. Catal. B Environ. 144, 189–195 (2014)
L.G. Devi, N. Kottam, B.N. Murthy, S.G. Kumar, J. Mol. Catal. A Chem. 328, 44–52 (2010)
F. Jiang, Z. Zheng, Z.Y. Xu, S.R. Zheng, Z.B. Guo, L.Q. Chen, J. Hazard. Mater. 134, 94–103 (2006)
S.K. Samantaray, P. Mohapatra, K. Parida, J. Mol. Catal. A Chem. 198, 277–287 (2003)
N.S. Begum, H.M.F. Ahmed, K.R. Gunashekar, Bull. Mater. Sci. 31, 747–751 (2008)
P.P. Hankare, R.P. Patil, A.V. Jadhav, R.S. Pandav, K.M. Garadkar, R. Sasikala, A.K. Tripathi, J. Alloys Compd. 509, 2160–2163 (2011)
P. Priyadharsini, A. Pradeep, P. Sambasiva Rao, G. Chandrasekaran, Mater. Chem. Phys. 116, 207–213 (2009)
P. Prieto, V. Nistor, K. Nouneh, M. Oyama, M. Abd-Lefdil, R. Diaz, Appl. Surf. Sci. 258, 8807–8813 (2012)
D.F. Ollis, E. Pelizzetti, N. Serpone, Environ. Sci. Technol. 25, 1523–1529 (1991)
I.K. Konstantinou, T.A. Albanis, Appl. Catal. B Environ. 49, 1–14 (2004)
K. Rajeshwar, J. Appl. Electrochem. 25, 1067–1082 (1995)
J.R. Bolton, K.G. Bircher, W. Tumas, C.A. Tolman, Pure Appl. Chem. 73, 627–637 (2001)
J. Saien, A. Azizi, A.R. Soleymani, J. Iran. Chem. Soc. 11, 1439–1448 (2014)
US Government Energy Information Administration, Independent Statistics and Analysis (2015). http://www.eia.doe.gov
Acknowledgments
The authors wish to thank the university authorities for providing the financial support to carry out this project. The authors also acknowledge Evonik industries for supplying TiO2 P-25 as a gift for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saien, J., Ghamari, F. & Azizi, A. The role of counter-anions in photocatalytic reduction of Ni(II) with a trace amount of titania nanoparticles. J IRAN CHEM SOC 13, 2247–2255 (2016). https://doi.org/10.1007/s13738-016-0943-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0943-6