Abstract
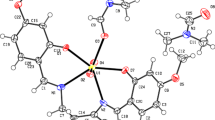
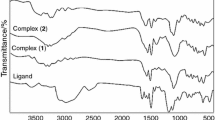
Uranyl(VI) complexes, [UO2(X-saloph)(solvent)], where saloph denotes N,N’-bis(salicylidene)-1,2-phenylenediamine and X = NO2, Cl, Me, H; were synthesized and characterized by 1H NMR, IR, UV–Vis spectroscopy, thermal gravimetry (TG), cyclic voltammetry, elemental analysis (C.H.N) and X-ray crystallography. X-ray crystallography of [UO2(4-nitro-saloph)(DMF)] revealed coordination of the uranyl by the tetradentate Schiff base ligand and one solvent molecule, resulting in seven-coordinated uranium. The complex of [UO2(4-nitro-saloph)(DMF)] was also synthesized in nano form. Transmission electron microscopy image showed nano-particles with sizes between 30 and 35 nm. The TG method and analysis of Coats-Redfern plots revealed that the kinetics of thermal decomposition of the complexes is of the first-order in all stages. The kinetics and mechanism of the exchange reaction of the coordinated solvent with tributylphosphine was investigated by spectrophotometric method. The second-order rate constants at four temperatures and the activation parameters showed an associative mechanism for all corresponding complexes with the following trend: 4-Nitro > 4-Cl > H > 4-Me. It was concluded that the steric and electronic properties of the complexes were important for the reaction rate. For analysis of anticancer properties of uranyl Schiff base complexes, cell culture and MTT assay was carried out. These results showed a reduction of jurkat cell line concentration across the complexes.










Similar content being viewed by others
References
V.P. Lozitsky, V.E. Kuzmin, A.G. Artemenko, R.N. Lozitska, A.S. Fedtchouk, E.N. Muratov, A.K. Mescheriakov, SAR QSAR Environ. Res. 16, 219 (2005)
D. Sinha, A.K. Tiwari, S. Singh, G. Shukla, P. Mishra, H. Chandra, A.K. Mishra, Eur. J. Med. Chem. 43, 160 (2008)
S. Adsule, V. Barve, D. Chen, F. Ahmed, Q.P. Dou, S. Padhye, F.H. Sarkar, J. Med. Chem. 49, 7242 (2006)
S. Ren, R. Wang, K. Komatsu, P. Bonaz-Krause, Y. Zyrianov, C.E. McKenna, C. Csipke, Z.A. Tokes, E.J. Lien, J. Med. Chem. 45, 410 (2002)
Z.H. Abd El-Wahab, M.R. El-Sarrag, Spectrochim. Acta Part A 60, 271 (2004)
E. Yoshida, S. Yamada, Bull. Chem. Soc. Jpn. 40, 1395 (1967)
A. Elmali, C.T. Zeyrek, Y. Elerman, T.N. Durlu, J. Chem. Crystallogr. 30, 167 (2000)
A.A. Soliman, W. Linert, Thermochim. Acta 338, 67 (1999)
S. Zolezzi, A. Decinti, E. Spodine, Polyhedron 18, 897 (1999)
G. Gordon, H. Taube, J. Inorg. Nucl. Chem. 16, 272 (1961)
W. Jung, Y. Ikeda, H. Tomiyasu, H. Fukutomi, Bull. Chem. Soc. Jpn. 57, 2317 (1984)
Y. Kato, H. Fukutomi, J. Inorg. Nucl. Chem. 38, 1323 (1976)
K. Okuyama, Y. Ishikawa, Y. Kato, H. Fukutomi, Bull. Res. Lab. Nucl. React. 3, 39 (1978)
S.F. Lincoln, Pure Appl. Chem. 51, 2059 (1979)
H. Tomiyasu, H. Fukutomi, Bull. Res. Lab. Nucl. React. 7, 57 (1982)
E. Comini, Anal. Chim. Acta 568, 28 (2006)
N. Kocak, M. Sahin, S. Kucukkolbasi, Z.O. Erdogan, Int. J. Biol. Macromol. 51, 1159 (2012)
H.L. Karlsson, J. Gustafsson, P. Cronholm, L. Moller, Toxicol. Lett. 188, 112 (2009)
L. Palatinus, G. Chapuis, J. Appl. Cryst. 40, 786 (2007)
V. Petricek, M. Dusek, L. Palatinus, Z. Kristallogr. 229, 345 (2014)
T. Mossman, J. Immunol. Methods 65, 55 (1983)
D.J. Evans, P.C. Junk, M.K. Smith, Polyhedron 21, 2421 (2002)
K. Mizuoka, Y. Ikeda, Inorg. Chem. 42, 3396 (2003)
S.Y. Ebrahimipour, J.T. Mague, A. Akbari, R. Takjoo, J. Mol. Struct. 1028, 148 (2012)
M. Ebel, D. Rehder, Inorg. Chem. 45, 7083 (2006)
D.N. Kumar, B.S. Garg, Spectrochim. Acta, Part A 64, 141 (2006)
U. Casellato, S. Tamburini, P. Tomasin, P.A. Vigato, Inorg. Chim. Acta 341, 118 (2002)
M.S. Bharara, K. Heflin, S. Tonks, K.L. Strawbridge, A. E. V. Gorden. Dalton Trans. 10, 2966 (2008)
A.H. Kianfar, M. Dostani, Spectrochim. Acta, Part A 82, 69 (2011)
Z. Asadi, F. Golzard, V. Eigner, M. Dusek, J. Coord. Chem. 66, 3629 (2013)
M.S. Refat, M.Y. El-Sayed, A.M.A. Adam, J. Mol. Struct. 1038, 62 (2013)
Z. Asadi, M.R. Shorkaei, Spectrochim. Acta, Part A 105, 344 (2013)
A.W. Coats, J.P. Redfern, Nature 201, 68 (1964)
Z. Asadi, M. Asadi, F.D. Firuzabadi, R. Yousefi, M. Jamshidi, J. Iran. Chem. Soc. 11, 423 (2014)
H.C. Hardwick, D.S. Royal, M. Helliwell, S.J.A. Pope, L. Ashton, R. Goodacred, C.A. Sharrad, Dalton Trans. 40, 5939 (2011)
Z. Asadi, M. Asadi, F.D. Firuzabadi, Int. J. Chem. Kinet. 45, 795 (2013)
Acknowledgments
We are grateful to Shiraz University Research Council for its financial support. The crystallographic part was supported by the project 14-03276S of the Czech Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: Supplementary material
Appendix 1: Supplementary material
CCDC 914883 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Rights and permissions
About this article
Cite this article
Asadi, Z., Nasrollahi, R., Dusek, M. et al. Effect of the substitutional groups on the electrochemistry, kinetic of thermal decomposition and kinetic of substitution of some uranyl Schiff base complexes. J IRAN CHEM SOC 13, 913–924 (2016). https://doi.org/10.1007/s13738-016-0807-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0807-0