Abstract
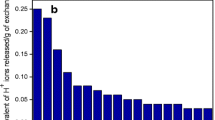
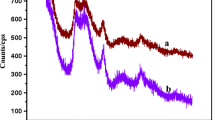
A new surfactant-based nanocomposite cation exchanger, sodium dodecyl sulfate–Th(IV) tungstate (SDS–TT) was prepared by the sol–gel method. The SDS–TT was characterized by FTIR, XRD, TGA, SEM, EDS and TEM. The distribution studies for various metal ions on SDS–TT were performed in different acidic mediums. On the basis of distribution coefficient values, SDS–TT was found to be selective for Cu2+ metal ion. SDS–TT was successfully used for the quantitative separation of Cu2+ from the synthetic mixture, pharmaceutical formulation and brass sample. The regeneration studies were performed which showed a decrease in the recovery of Cu2+ from 88 to 70 % after seven consecutive cycles.






Similar content being viewed by others
References
J. Keary, A.D. Mortimer, in Ion Exchange Developments and Applications, vol. 35, ed. by J.A. Greig (The Royal Society of Chemistry, Cambridge, UK, 1996), pp. 35–42
J. Lee, D.F. Hussey, G.L. Foutch, in Ion Exchange at the Millennium, vol. 61 ed. by J.A. Greig (Imperial College Press, London, 2000) pp. 61–68
A. Clearfield, Inorganic Ion Exchange Materials (CRC Press, Boca Raton, 1982)
Mu. Naushad, Chem. Eng. J. 235, 100 (2014)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 171, 456 (2011)
S.S.M. Hassan, S.A. Marei, I.H. Badr, H.A. Arida, Anal. Chim. Acta 427, 21 (2001)
S.A. Nabi, S.A. Ganai, M. Naushad, Ads. Sci. Tech. 26, 463 (2009)
G. Sharma, D. Pathania, Mu. Naushad, N.C. Kothiyal. Chem. Eng. J. 251, 413 (2014)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 166, 639 (2011)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 169, 38 (2011)
S.A. Nabi, Mu. Naushad, Inamuddin, J. Hazard Mater. 42, 404 (2007)
S.A. Nabi, Mu. Naushad, Chem. Eng. J. 158, 100 (2010)
O.M. Vatutsina, V.S. Soldatov, V.I. Sokolova, J. Johann, M. Bissen, A. Weisswnbacher, React. Funct. Polym. 67, 184 (2007)
L. Wang, L. Yang, Y. Li, Y. Zhang, X. Ma, Z. Ye, Chem. Eng. J. 163, 364 (2010)
L.C. Lin, R.S. Juang, Chem. Eng. J. 132, 205 (2007)
J. Peric, M. Trgo, N.V. Medvidovic, Water Res. 38, 1893 (2004)
Mu. Naushad, Inamuddin, T.A. Rangreez, Z.A. ALOthman, J. Electroanal. Chem. 713, 125 (2014)
Z. Ujang, G.K. Anderson, Water Sci. Technol. 38, 521 (1998)
A. Dabrowski, Z. Hubicki, P. Podkoscielny, E. Robens, Chemosphere 56, 91 (2004)
M.R. Awual, M.M. Hasan, A. Shahat, M. Naushad, H. Shiwaku, T. Yaita, Chem. Eng. J. 265, 210 (2015)
Z.A. ALOthman, M.M. Alam, Mu. Naushad, J. Ind. Eng. Chem. 19, 956 (2013)
K.G. Varshney, M.Z.A. Rafiquee, A. Somya, M. Drabik, Indian J. Chem. 45A, 1856 (2006)
K.G. Varshney, M.Z.A. Rafiquee, A. Somya, Coll. Surf A Physiochem. Eng. Asp. 301, 224 (2007)
N. Iqbal, M. Mobin, M.Z.A. Rafiquee, Chem. Eng. J. 169, 43 (2011)
K.M. El-Azony, A.M. Ismail, A.A. El-Mohty, J. Radioanal. Nucl. Chem. 289, 381 (2011)
A. Mohammad, Inamuddin, A. Amin, J. Therm. Anal. Calorim. 107, 127 (2012)
L.L. Schramm, E.N. Stasiuk, D.G. Marangoni, Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 99, 3 (2003)
S. Tagashira, S. Kimoto, K. Nozaki, Y. Murakami, Anal. Sci. 25, 723 (2009)
N. Li, N. Bai, Sep. Purif. Technol. 42, 237 (2005)
M.M. Rao, A. Ramesh, G.P.C. Rao, K. Seshaiah, J. Hazard Mater. B 129, 123 (2006)
S.A. Nabi, S. Usmani, N. Rahman, Ann. Chim. Sci. Fr. 21, 521 (1996)
W. Kossel, Ann. Phys. 49, 229 (1916)
L. Paulling, J. Am. Chem. Soc. 51, 1010 (1929)
F.A. Miller, C.H. Wilkins, Anal. Chem. 24, 1253 (1952)
S.A. Nabi, M. Naushad, Coll Surf. A Phys. Eng. Asp. 293, 175 (2007)
C. Dual, Inorganic thermogravimetric analysis (Elsevier, Amsterdam, 1963)
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naushad, M., ALOthman, Z.A., Alam, M.M. et al. Synthesis of sodium dodecyl sulfate-supported nanocomposite cation exchanger: removal and recovery of Cu2+ from synthetic, pharmaceutical and alloy samples. J IRAN CHEM SOC 12, 1677–1686 (2015). https://doi.org/10.1007/s13738-015-0642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0642-8