Abstract
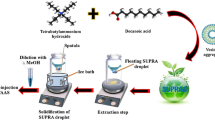
Solidified floating organic drop microextraction was applied as a separation/preconcentration step prior to the electrothermal atomic absorption spectrometric (ETAAS) determination of ultra trace of antimony species. The method was based on the formation of an extractable complex between Sb(III) and ammonium pyrrolidinedithiocarbamate at pH ~ 5, while Sb(V) was remained in the aqueous phase. The antimony extracted into 1-undecanol was determined by ETAAS. Total antimony was determined after the reduction of Sb(V) to Sb(III) with potassium iodide and ascorbic acid. The amount of Sb(V) was determined from the difference of concentration of total antimony and Sb(III). Under the optimum conditions an enhancement factor of 437.5 and a detection limit of 5.0 ng L−1for the preconcentration of 25 mL of sample was achieved. The relative standard deviation at 300 ng L−1 of antimony was found to be 3.5 % (n = 6). The proposed method was successfully applied to the determination of antimony in tea, basil and natural water samples.




Similar content being viewed by others
References
N. Furuta, A. Iijima, A. Kambe, K. Sakai, K. Sato, J. Environ. Monit. 7, 1155 (2005)
I. Palchetti, A. Cagnini, M. Mascini, A.P.F. Turner, Microchim. Acta 131, 65 (1999)
P. Smichowski, Talanta 75, 2 (2008)
H.R. Hansen, S.A. Pergantis Anal, Chemistry 79, 5304 (2007)
M. Krachler, H. Emons, J. Zheng, Trends Anal. Chem. 20, 79 (2001)
S. Saracoglu, M. Soylak, M. Dogan, L. Elci, Anal. Sci. 19, 259 (2003)
R. Poon, I. Chu, P. Lecavalier, V.E. Valli, W. Foster, S. Gupta, B. Thomas, Food Chem. Toxicol. 36, 21 (1998)
C. Bosch Ojeda, F. Sánchez Rojas, J.M. Cano Pavón, L. Terrer Martín, Anal. Bioanal. Chem. 382, 513 (2005)
IARC. Antimony trioxide and antimony trisulfide. IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Printing (Vol. 47, International Agency for Research on Cancer, Lyon, France, pp. 291–305) (1989)
G. Gillain, Talanta 29, 651 (1982)
N. Ulrich, Speciation of Antimony, in Handbook of Elemental Speciation II—Species in the Environment, Food, Medicine and Occupational Health, ed. by R. Cornelis (Wiley, Chichester, 2005)
N. Ozdemir, M. Soylak, L. Elci, M. Dogan, Anal. Chim. Acta 505, 37 (2004)
K. Zih-Perényi, P. Jankovics, E. Sugár, A. Lásztity, Spectrochim. Acta, Part B 63, 445 (2008)
I. De Gregori, W. Quiroz, H. Pinochet, F. Pannier, M. Potin-Gautier, J. Chromatogr. A 1091, 94 (2005)
C.Z. Huang, B. Hu, Z.C. Jiang, Spectrochim. Acta, Part B 62, 454 (2007)
Y. Morita, T. Kobayashi, T. Kuroiwa, T. Narukawa, Talanta 73, 81 (2007)
G. Capodaglio, C.M.G. Van Den Berg, G. Scarponi, J. Electroanal. Chem. Interfacial Electrochem. 235, 275 (1987)
H. Wu, X. Wang, B. Liu, Y. Liu, S. Li, J. Lu, J. Tian, W. Zhao, Spectrochim. Acta, Part B 66, 74 (2011)
C.H. Chung, E. Iwamoto, M. Yamamoto, Y. Yamamoto, Spectrochim. Acta, Part B 39, 459 (1984)
Y.C. Sun, J.Y. Yang, Y.F. Lin, M.H. Yang, Z.B. Alfassi, Anal. Chim. Acta 276, 33 (1993)
X. Jiang, S. Wen, G. Xiang, J. Hazard. Mater. 175, 146 (2010)
P.J. Craig, T. Sergeeva, R.O. Jenkins, Microchim. Acta 137, 221 (2001)
M. Shamsipur, M. Ramazani, Talanta 75, 294 (2008)
M. Kaykhaii, A. Khatibi, J. Iran. Chem. Soc. 8, 374 (2011)
N. Gouharzi, J. Agric. Food Chem. 57, 1099 (2009)
M.R. Khalili Zanjani, Y. Yamini, S. Shariati, J.A. Jonsson, Anal. Chim. Acta 585, 286 (2007)
S. Dadfarnia, A.M. Salmanzadeh, A.M. Haji Shabani, Anal. Chim. Acta 623, 163 (2008)
M. Shirani Bidabadi, S. Dadfarnia, A.M. Haji Shabani, J. Hazard. Mater. 166, 291 (2009)
S. Dadfrania, A.M. Haji Shabani, Anal. Chim. Acta 658, 107 (2010)
C.A. Şahin, I.A. Tokgöz, Anal. Chim. Acta 667, 83 (2010)
M. Rohani Moghadam, S. Dadfarnia, A.M. Haji Shabani, J. Hazard. Mater. 186, 169 (2010)
Z. Fan, Anal. Chim. Acta 585, 300 (2007)
R. Rivas, I. López-García, M. Hernández-Córdoba, Spectrochim. Acta, Part B 64, 329 (2009)
S.R. Yousefi, F. Shemirani, M.R. Jamali, Anal. Lett. 43, 2563 (2010)
J.M. Serafimovska, S. Arpadjan, T. Stafilov, Michrochem. J 99, 46 (2011)
F. Pena-Pereira, I. Lavilla, C. Bendicho, Microchim. Acta 164, 77 (2009)
M. Korenovska, J. Food Nutr. Res. 45, 84 (2006)
M. Mohamadi, A. Mostafavi, Talanta 81, 309 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dadfarnia, S., Haji Shabani, A.M. & Nili Ahmad abadi, M. Solidified floating organic drop microextraction–electrothermal atomic absorption spectrometry for ultra trace determination of antimony species in tea, basil and water samples. J IRAN CHEM SOC 10, 289–296 (2013). https://doi.org/10.1007/s13738-012-0157-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0157-5