Abstract
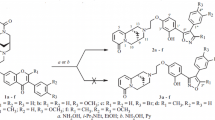
A new series of N-hydroxyethylpyrazole (12a–f) and N-hydroxymethylpyrazole derivatives (15a–f) were designed for their estrogenic activities, having a 11.0 ± 0.5 Å distance between their two hydroxyl groups, aliphatic–OH and phenolic–OH similar to 17β-estradiol (E2) as an endogenous hormone. To synthesize the title compounds, the key intermediate 1,3-dicarbonyl derivatives (2 and 8), were treated with hydrazine hydrate to produce the pyrazole ring 5 and 9. Further hydroxyalkylation of the latter produced the title pyrazoles. The position of hydroxyethyl or hydroxymethyl substituents in the products was determined through 2D NOE NMR spectroscopy.





Similar content being viewed by others
References
S.W. Landvatter, J.A. Katzenellenbogen, J. Med. Chem. 25, 1300 (1982)
S. Bertini, A. De Cupertinis, C. Granchi, B. Bargagli, T. Tuccinardi, A. Martinelli, M. Macchia, J.R. Gunther, K.E. Carlson, J.A. Katzenellenbogen, F. Minutolo, Eur. J. Med. Chem. 46, 2453 (2011)
R.E. McDevitt, M.S. Malamas, E.S. Manas, R.J. Unwalla, Z.B. Xu, C.P. Miller, H.A. Harris, Bioorg. Med. Chem. Lett. 15, 3137 (2005)
M. Waibel, K.J. Kieser, K.E. Carlson, F. Stossi, B.S. Katzenellenbogen, J.A. Katzenellenbogen, Eur. J. Med. Chem. 44, 3560 (2009)
R.A. Magarian, L.B. Overaacre, S. Singh, Curr. Med. Chem. 1, 61 (1994)
J. Grundy, Chem. Rev. 57, 281 (1957)
D.R. Compton, K.E. Carlson, J.A. Katzenellenbogen, Bioorg. Med. Chem. Lett. 14, 5681 (2004)
M. Shekarchi, M. Pirali-Hamedani, L. Navidpour, N. Adib, A. Shafiee, J. Iran. Chem. Soc. 5, 150 (2008)
S.R. Stauffer, C.J. Coletta, R. Tedesco, G. Nishigushi, K. Carlson, J. Sun, B.S. Katzenellenbogen, J.A. Katzenellenbogan, J. Med. Chem. 43, 4934 (2000)
B.E. Fink, D.S. Mortensen, S.R. Stauffer, Z.D. Aron, J.A. Katzenellenbogen, Chem. Biol. 6, 205 (1999)
S.R. Stauffer, Y.R. Huang, Z.D. Aron, C.J. Coletta, J. Sun, B.S. Katzenellenbogen, J.A. Katzenellenbogen, Bioorg. Med. Chem. 9, 151 (2001)
X. Alexi, K.M. Kasiotis, N. Fokialakis, G. Lambrinidis, A.K. meligova, E. Mikros, S.A. Haroutounian, M.N. Alexis. J. steroid Biochem. Mol. Biol. 117, 159 (2009)
J.S. Wright, H. Shadnia, J.M. Anderson, J.M. Anderson, J.M. Anderson, T. Durst, M. Assim, M. El-salfiti, C. Choueiri, M.A.C. Pratt, S.C. Ruddy, R. Lau, K.E. Carlson, J.A. Katzenellenbogen, P.J. O’Brien, L. Wan, J. Med. Chem. 54, 433 (2011)
N. Minami, Y. Suzuki, Yakugaku Zasshi 95, 815 (1975)
M.T. Di Parsia, C. Suarez, M.J. Vitolo, V.E. Marquez, J. Med. Chem. 24, 117 (1981)
E.C. Taylor, A. Mc Killop, Y. Shvo, G.H. Hawks, Tetrahedron 23, 2081 (1967)
J.G. Morgan, K. Darrell Berlin, N.N. Durham, R.W. Chesnut, J. Heterocycl. Chem. 8, 61 (1971)
J.A. may, P.W. Zinke, US Pat., 2003, 2003/83346
Z.-F. Tao, G, Li, Y. Tong, Z. Chen, P. Merta, P. Kovar, H. Zhang, S.H. Rosenberg, H.L. Sham, T.J. Sowin, N.-H. Lin, Bioorg. Med. Chem. Lett. 17, 4308 (2007)
V.K. Aggarwal, J. de Vicente, R.V. Bonnert, J. Org. Chem. 68, 5381 (2003)
L. Levi, C. Boersch, C.F. Gers, E. Merkul, T.J.J. Müller, Molecules 16, 9340 (2011)
C.J. Valduga, H.S. Braibante, M.E.F. Braibante, J. Heterocycl. Chem. 34, 1453 (1997)
R.G. Parker, J.D. Roberts, J. Org. Chem. 35, 996 (1970)
S. Sivaprasad, R. Sridhar, P.T. Perumal, J. Heterocycl. Chem. 43, 389 (2006)
A.O. Baltayan, V.I. Rstakyan, S.K. Antanosyan, F.S. Kinoyan, O.S. Attaryan, G.V. Asratyan, Russ. J. Gen. Chem. 79, 2417 (2009)
K.M. Kasiotis, N. Fokialakis, S.A. Haroutounian, Synthesis 11, 1791 (2006)
A.R. Katritzky, P. Lue, K. Akutagawa, Tetrahedron 45, 4253 (1989)
Acknowledgments
This research was supported by a grant from National Elite Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Allahyari-Devin, M., Abedi, B., Navidpour, L. et al. Synthesis of aryl-substituted or aryl-fused N-hydroxyethyl and N-hydroxymethypyrazole derivatives as potential ligands for the estrogen receptor. J IRAN CHEM SOC 10, 43–53 (2013). https://doi.org/10.1007/s13738-012-0126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0126-z