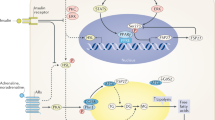
Abstract
Androgens are regulators of important adipocyte functions such as adipogenesis, lipid storage, and lipolysis. Through depot-specific impact on the cells of each fat compartment, androgens could modulate body fat distribution patterns in humans. Testosterone and dihydrotestosterone have been shown to inhibit the differentiation of preadipocytes to lipid-storing adipocytes in several models including primary cultures of human adipocytes from both men and women. Androgen effects have also been observed on some markers of lipid metabolism such as LPL activity, fatty acid uptake, and lipolysis. Possible depot-specific and sex-specific effects have been observed in some but not all models. Transformation of androgen precursors to active androgens or their inactivation by enzymes that are expressed and functional in adipose tissue may contribute to modulate the local availability of active hormones. These phenomena, along with putative depot-specific interactions with glucocorticoids may contribute to human body fat distribution patterns.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tran BX, Nair AV, Kuhle S, Ohinmaa A, Veugelers PJ. Cost analyses of obesity in Canada: scope, quality, and implications. Cost Eff Resour Allocation C/E. 2013;11:3.
Yang Z, Zhang N. The Burden of Overweight and Obesity on Long-term Care and Medicaid Financing. Med Care. 2014;52:658–63.
Veilleux A, Tchernof A. Sex differrences in body fat distribution. In: Symonds ME, editor. Adipose Tissue Biology. 1st ed. Nottingham: Springer; 2012. p. 123–66.
Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7.
Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–30.
Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, et al. Puberty and body composition. Horm Res. 2003;60:36–45.
Van Loan MD. Total body composition: birth to old age. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human body composition. Champaign: Human Kinetics; 1996. p. 205–15.
Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404.
Tchernof A. Sex differences in energy balance, body composition and body fat distribution. In: Brown FM, Wyckoff J, Tsatsoulis A, editors. Diabetes in women. Heidelberg: Springer; 2009.
Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. JCEM. 2012;97:1517–25.
Borel AL, Nazare JA, Smith J, Almeras N, Tremblay A, Bergeron J, et al. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity. 2012;20:1223–33.
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
Bergman RN, Van Citters GW, Mittelman SD, Dea MK, Hamilton-Wessler M, Kim SP, et al. Central role of the adipocyte in the metabolic syndrome. J Investig Med. 2001;49:119–26.
Mauriège P, Galitzky J, Berlan M, Lafontan M. Heterogeneous distribution of beta and alpha-2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. Eur J Clin Invest. 1987;17:156–65.
Hellmér J, Marcus C, Sonnenfeld T, Arner P. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992;75:15–20.
Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–8.
Tchernof A, Bélanger C, Morisset AS, Richard C, Mailloux J, Laberge P, et al. Regional differences in adipose tissue metabolism in women: Minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353–60.
Björntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–6.
Guo Z, Jensen MD. Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol. 1998;84:1674–9.
Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab. 2001;280:E1000–6.
Jensen MD, Cardin S, Edgerton D, Cherrington A. Splanchnic free fatty acid kinetics. Am J Physiol Endocrinol Metab. 2003;284:E1140–8.
Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–12.
Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes. 2008;32:283–91.
Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–31.
Carpentier AC, Labbé SM, Grenier-Larouche T, Noll C. Abnormal dietary fatty acid metabolic partitioning in insulin resistance and Type 2 diabetes. Clin Lipidol. 2013;6:703–16.
Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–81.
Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800.
Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14.
Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64:163–9.
Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue - an update. Horm Metab Res. 2007;39:314–21.
Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46.
Bain J. The many faces of testosterone. Clin Interv Aging. 2007;2:567–76.
Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–83.
Matsumoto T, Shiina H, Kawano H, Sato T, Kato S. Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol. 2008;109:236–41.
Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–8.
Veilleux A, Cote JA, Blouin K, Nadeau M, Pelletier M, Marceau P, et al. Glucocorticoid-induced androgen inactivation by aldo-keto reductase 1C2 promotes adipogenesis in human preadipocytes. Am J Physiol Endocrinol Metab. 2012;302:E941–9. This study describes the stimulatory effect of glucocorticoids on DHT inactivation in adipose tissue and demonstrates that 3α-HSD-3 (AKR1C2) is responsible for this phenomenon.
Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148.
Blouin K, Després JP, Couillard C, Tremblay A, Prud'homme D, Bouchard C, et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism. 2005;54:1034–40.
Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)–a precursor steroid or an active hormone in human physiology. J Sex Med. 2011;8:2960–82.
Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. 2004;151:1–14.
Côté JA, Lessard J, Mailloux J, Laberge PY, Rhéaume C, Tchernof A. Circulating 5 α -dihydrotestosterone, abdominal obesity, and adipocyte characteristics in women. Horm Mol Biol Clin Invest. 2012;12:391–400.
Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108:272–80.
Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–4.
Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. 2002;11:1041–7.
Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41.
Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76.
Seftel A. Male hypogonadism. Part II: etiology, pathophysiology, and diagnosis. Int J Impot Res. 2006;18:223–8.
Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7.
Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–29. This study demonstrates that long-term testosterone therapy ameliorates features of the metabolic syndrome and can be clinically useful through such reduction of cardiometabolic risk in hypogonadal men.
Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515. This study shows that long-term testosterone therapy resulted in significant improvements of cardiometabolic risk factors and produced clinical benefits in obese, diabetic men.
Mårin P, Holmäng S, Jönsson L, Sjöström L, Kvist H, Holm G, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged and obese men. Int J Obesity. 1992;16:991–7.
Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, et al. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab. 2004;89:718–26.
Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–15.
Elbers JMH, Asscheman H, Seidell JC, Megens JA, Gooren LJG. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82:2044–7.
Elbers JMH, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol. 2003;58:562–71.
Glazer G. Atherogenic effects of anabolic steroids on serum lipid levels. A literature review. Arch Intern Med. 1991;151:1925–33.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800.
Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:999–1004.
Casson PR, Toth MJ, Johnson JV, Stanczyk FZ, Casey CL, Dixon ME. Correlation of serum androgens with anthropometric and metabolic indices in healthy, nonobese postmenopausal women. J Clin Endocrinol Metab. 2010;95:4276–82.
Keller JL, Casson PR, Toth MJ. Relationship of androgens to body composition, energy and substrate metabolism and aerobic capacity in healthy, young women. Steroids. 2011;76:1247–51.
Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–8.
Cao Y, Zhang S, Zou S, Xia X. The relationship between endogenous androgens and body fat distribution in early and late postmenopausal women. PLoS One. 2013;8:e58448.
Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone- binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–6.
Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE family study. J Clin Endocrinol Metab. 2000;85:1026–31.
Tchernof A, Després JP, Bélanger A, Dupont A, Prud'homme D, Moorjani S, et al. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism. 1995;44:513–9.
de Pergola G, Triggiani V, Giorgino F, Cospite MR, Garruti G, Cignarelli M, et al. The free testosterone to dehydroepiandrosterone sulphate molar ratio as a marker of visceral fat accumulation in premenopausal obese women. Int J Obes. 1994;18:659–64.
De Simone M, Verrotti A, Lughetti L, Palumbo M, Farello G, Di Cesare E, et al. Increased visceral adipose tissue is associated with increased circulating insulin and decreased sex hormone binding globulin levels in massively obese adolescent girls. J Endocrinol Invest. 2001;24:438–44.
Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1996;81:59–64.
Williams DP, Boyden TW, Pamenter RW, Lohman TG, Going SB. Relationship of body fat percentage and fat distribution with dehydroepiandrosterone sulfate in premenopausal females. J Clin Endocrinol Metab. 1993;77:80–5.
Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, et al. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an italian study on healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab. 1996;81:1173–8.
O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143c:277–84.
Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: Recent advances. Mol Cell Endocrinol. 2009;301:97–103.
Veilleux A, Blouin K, Tchernof A. Mechanisms of androgenic action in adipose tissue. Clin Lipidol. 2009;4:367–78.
Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cel Biol. 2013;92:229–36.
Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44.
Huang CK, Tsai MY, Luo J, Kang HY, Lee SO, Chang C. Suppression of androgen receptor enhances the self-renewal of mesenchymal stem cells through elevated expression of EGFR. Biochim Biophys Acta. 1833;2013:1222–34.
Fujioka K, Kajita K, Wu Z, Hanamoto T, Ikeda T, Mori I, et al. Dehydroepiandrosterone reduces preadipocyte proliferation via androgen receptor. Am J Physiol Endocrinol Metab. 2012;302:E694–704.
Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, et al. Testosterone inhibits adipogenic differentiation in 3 T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–54.
Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10 T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–8.
Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141:649–56.
Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–6.
McNelis JC, Manolopoulos KN, Gathercole LL, Bujalska IJ, Stewart PM, Tomlinson JW, et al. Dehydroepiandrosterone exerts antiglucocorticoid action on human preadipocyte proliferation, differentiation, and glucose uptake. Am J Physiol Endocrinol Metab. 2013;305:E1134–44.
Blouin K, Nadeau M, Perreault M, Drolet R, Marceau P, Mailloux J, et al. Effects of Androgens on Adipocyte Differentiation and Adipose Tissue Explant Metabolism in Men and Women. Clin Endocrinol. 2009;72:176–88.
Gupta V, Bhasin S, Guo W, Singh R, Miki R, Choong K, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40.
Garcia E, Lacasa M, Agli B, Giudicelli Y, Lacasa D. Modulation of rat preadipocyte adipose conversion by androgenic status: involvement of C/EBPs transcription factors. J Endocrinol. 1999;161:89–97.
Barbosa-Desongles A, Hernandez C, Simo R, Selva DM. Testosterone induces cell proliferation and cell cycle gene overexpression in human visceral preadipocytes. Am J Physiol Cell Physiol. 2013;305:C355–9.
Rodriguez-Cuenca S, Monjo M, Proenza AM, Roca P. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am J Physiol Endocrinol Metab. 2005;288:E200–7.
Kolditz CI, Langin D. Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care. 2010;13:377–81.
Chaves VE, Frasson D, Kawashita NH. Several agents and pathways regulate lipolysis in adipocytes. Biochimie. 2011;93:1631–40.
Zierath JR, Livingston JN, Thorne A, Bolinder J, Reynisdottir S, Lonnqvist F, et al. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway. Diabetologia. 1998;41:1343–54.
Rebuffé-Scrive M, Mårin P, Björntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–5.
Xu X, de Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–34.
Hernandez-Morante JJ, Perez-de-Heredia F, Lujan JA, Zamora S, Garaulet M. Role of DHEA-S on body fat distribution: gender- and depot-specific stimulation of adipose tissue lipolysis. Steroids. 2008;73:209–15.
Varlamov O, Chu MP, McGee WK, Cameron JL, O'Rourke RW, Meyer KA, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154:4126–35.
Dicker A, Ryden M, Näslund E, Muehlen IE, Wiren M, Lafontan M, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–8.
Anderson LA, McTernan PG, Harte AL, Barnett AH, Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diab Obes Metab. 2002;4:209–13.
Pecquery R, Dieudonne MN, Cloix JF, Leneveu MC, Dausse JP, Giudicelli Y. Enhancement of the expression of the α2-adrenoreceptor protein and mRNA by a direct effect of androgens in white adipocytes. Biochem Biophys Res Commun. 1995;206:112–8.
Pecquery R, Leneveu MC, Giudicelli Y. Influence of androgenic status on the alpha 2/beta-adrenergic control of lipolysis in white fat cells: predominant alpha 2-antilipolytic response in testosterone-treated-castrated hamsters. Endocrinology. 1988;122:2590–6.
Xu XF, de Pergola G, Björntorp P. Testosterone increases lipolysis and the number of beta- adrenoceptors in male rat adipocytes. Endocrinology. 1991;128:379–82.
Bélanger C, Hould FS, Lebel S, Biron S, Brochu G, Tchernof A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71:674–82.
Li M, Björntorp P. Effects of testosterone on triglyceride uptake and mobilization in different adipose tissues in male rats in vivo. Obesity Res. 1995;3:113–9.
Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153:3100–10.
Blouin K, Richard C, Bélanger C, Dupont P, Daris M, Laberge P, et al. Local androgen inactivation in abdominal visceral adipose tissue. J Clin Endocrinol Metab. 2003;88:5944–50.
Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, et al. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. 2006;191:637–49.
Blouin K, Blanchette S, Richard C, Dupont P, Luu-The V, Tchernof A. Expression and activity of steroid aldoketoreductases 1C in omental adipose tissue as positive correlates of adiposity in women. Am J Physiol Endocrinol Metab. 2005;288:E398–404.
Blouin K, Nadeau M, Mailloux J, Daris M, Lebel S, Luu-The V, et al. Pathways of Adipose Tissue Androgen Metabolism in Women: Depot Differences and Modulation by Adipogenesis. Am J Physiol Endocrinol Metab. 2009;296:E244–55.
Bujalska IJ, Walker EA, Tomlinson JW, Hewison M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1 in differentiating omental human preadipocytes: from de-activation to generation of cortisol. Endocr Res. 2002;28:449–61.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70.
Veilleux A, Rheaume C, Daris M, Luu-The V, Tchernof A. Omental adipose tissue type 1 11 beta-hydroxysteroid dehydrogenase oxoreductase activity, body fat distribution, and metabolic alterations in women. J CLin Endocrinol Metab. 2009;94:3550–7.
Hartig SM, He B, Newberg JY, Ochsner SA, Loose DS, Lanz RB, et al. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol. 2012;19:1126–41.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Studies from our group cited in this manuscript were funded by operating grants from the Canadian Institutes of Health Research to A Tchernof and Co-investigators (MOP-53195, MOP-102642 and MOP-130313).
Mouna Zerradi, Julie Dereumetz, Marie-Michèle Boulet declare that they have no conflict of interest.
André Tchernof declares research funding obtained from Johnson & Johnson (Ethicon Endosurgery) for projects on bariatric surgery, unrelated to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zerradi, M., Dereumetz, J., Boulet, MM. et al. Androgens, body fat Distribution and Adipogenesis. Curr Obes Rep 3, 396–403 (2014). https://doi.org/10.1007/s13679-014-0119-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-014-0119-6