Abstract
Obesity is the result of complex interactions of multiple factors that have gradually led to enduring changes in lifestyles, and thus, creating a global epidemic of major health concerns. The roles of genetics and the environment are vital and need to be explored to further our understanding of the etiology of childhood obesity. This review critically looked at published reports over the past decade on factors that are unmodifiable, such as genetics, ethnic differences, gestational weight and intrauterine conditions; as well as modifiable factors, such as socioeconomic status, diet, physical activity, sleep, and parental determinants. With the worldwide increase in prevalence of pediatric obesity over the past several decades, it is imperative that we understand the root causes of obesity in order to arrest the rising trend through better prevention and intervention strategies.
Similar content being viewed by others
Introduction
Childhood obesity is portrayed by the accrual of excessive body fat as well as the growth of excess adipocytes [1]. It is widely accepted as has been reported that overweight or obese children are at greater risk of becoming overweight or obese adults; who will face a life-time of increased risk for various diseases, including diabetes mellitus, cardiovascular disease, liver disease and certain cancers [2, 3]. Even during childhood, obesity has been reported to increase the risk for various medical problems, such as prediabetes and diabetes, metabolic syndrome, cardiovascular, pulmonary, orthopedic and gastrointestinal diseases, as well as psychological problems [3–5].
The incidence of overweight and obesity has great implications for public health. Globally, the prevalence of overweight and obesity has increased at an alarming rate. According to the World Health Organization (WHO), it is estimated that more than 40 million children under five years of age were overweight in year 2010, with close to 35 million of these children in developing countries [6]. Based on secular trends using the International Obesity Task Force (IOTF) criteria, Wang and Lobstein [7] estimated overweight to occur in 46% of school-aged children in the Americas, 41% in the Eastern Mediterranean region, 38% in the European region, 27% in the Western Pacific, and 22% in the Southeast Asia.
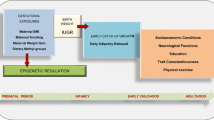
At the individual level, determinants of obesity include genetics, biology, behavior, and environments that foster an adverse balance between energy intake and energy expenditure [8]. However, there are also other relevant factors related to childhood obesity, including dietary status, physical activity, sleep duration, socio-economic status and intrauterine factors. It is therefore necessary to further explore the causal factors that contribute to obesity in children. Table 1 summarized the findings of recent studies on childhood obesity, while Fig. 1 presents the conceptual framework identifying several major modifiable and unmodifiable risk factors that may exert substantial influence on the etiology of childhood obesity.
Unmodifiable Risk Factors in Childhood Obesity
Genetic Influences
Genetic susceptibility to obesity is recognized as a highly heritable condition that begins at an early age. Genetic factors also determine whether one is susceptible to other obesity-related diseases [9]. Monogenic obesities have been examined extensively in children given that these conditions are rare, very severe, and generally begin in childhood. Candidate gene approaches have been used to identify the association between a variant or mutation within or near the candidate gene and a trait of interest (for example: obesity). With respect to single-gene mutation, a number of genes have been identified in individuals who were severely obese, namely pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin 1 (PCSK1), melanocortin-4 receptors (MC4R), corticotrophin-releasing hormone receptor (CRHR), leptin (LEP), leptin receptor (LEPR), cocaine- and amphetamine-regulated transcript (CART), brain-derived neurotrophic factor (BDNF) and single-minded homolog 1 (SIM-1) [10–16]. The defects in POMC produced altered peptide which is different from α-melanocyte-stimulating hormone (α-MSH) through PCSK1 and without α-MSH, MC4R is unable to play a role in weight regulation [10]. Deficiency of MC4R was found to associate with obesity by accelerated linear growth and increased final height as well as excess in insulin and partially suppress growth hormone [11]. Meanwhile, CRHR shows anorexigenic hypothalamic response by activation of urocortin to potently suppress food intake [12]. LEP and LEPR suppress the increase of CART [13] and prevent overeating by controlling the appetite [14]. BDNF have been implicated in the energy regulation by increasing gene expression for LEPR and POMC [15]. SIM1 are key factors interacting with the central melanocortin pathway in the control of appetite by decreasing MC4R [16]. However, cases of single-gene obesity cannot explain many others with latent genetic predisposition that is expressed only upon exposure to an obesogenic environment.
In most individuals, obesity results from the interaction of multiple genes that encode peptides that transmit hunger and satiety signals, regulate adipocyte growth and differentiation and control energy expenditure [17]. The Human Obesity Gene Map reported 253 loci from 61 genome-wide linkage scans, of which 15 loci have been replicated in at least three studies [18]. However, a meta-analysis by Saunders et al. showed that genome-wide linkage analysis may not be the best method for identifying genetic variants for obesity [19].
Genome-wide association studies (GWAS) have implicated many genetic loci for obesity in the past 5 years [20••], and of particular interest, the fat mass and obesity-associated protein (FTO) gene [21]. FTO gene expression in the hypothalamic arcuate nucleus was determined to contribute significantly to obesity as a consequence of hyperphagia, seemingly in the absence of changes in energy expenditure [22, 23]. Two other genes, TNNI3K and POMC, were identified by GWAS as being associated with childhood obesity [24]. In 2010, a study discovered two additional obesity loci (TNKS-MSRA and SDCCAG8) in extremely obese children and adolescents, with odds ratios of approximately 1.10 per risk allele for both loci for early-onset obesity [25].
Furthermore, gene-environment interactions also play an important role in childhood obesity [26, 27]. Differences in body composition is also a likely factor; specifically the lower abdominal adiposity found in African-American children compared with European or Hispanic American children [28, 29], and the higher trunk skinfold thickness in Chinese girls compared to Malaysian and Lebanese girls [30]. These differences in body composition phenotype suggest that the genetic makeup of individuals interacts with environmental factors from early developmental stages, as has been demonstrated by Fernandez et al. [26] and the twin and adoption study [31]. With the advent of next-generation sequencing techniques and advances in the field of exposomics, sensitive and specific tools to predict the obesity risk as early as possible are the challenges in the coming decade [32].
An environmental factor of major importance is nutrition. The risk of obesity is higher when an individual with a high-risk genetic profile is exposed to high-risk environment. For example, the Pima Indians have an inherent susceptibility to obesity, and obesity is more likely to develop among those living in Arizona as they are exposed to obesity-promoting environments, such as ‘Western diet’ and less exercise, compared with Pima Indians living in Mexico, who survive on traditional food and manual labor [33]. Moreover, obesity genes might also play a role in the choice and preference of dietary intake of saturated fat, carbohydrates, mono- and disaccharides, and polysaccharides [34]. A longitudinal study had suggested that high consumption of saturated fat increase the obesity risk associated with FTO gene [35]. To date, the genes responsible for individual differences in sensitivity to changes in energy balance have not all been identified. In view of the complexity of the biological systems involved in body weight regulation, these genes are likely to be numerous [36].
Di Castelnuovo et al. [37] suggested that positive assortative mating by BMI may assemble obesity-promoting risk alleles and probably undergo interaction between gene and environment in a family [38]. A recent paper revealed that weight changes over a period of two years was associated with marital status; partly due to shared environment, such as the stimulus to eat when dining together or the motivation for weight control to increase attractiveness [39]. Comparing children born to normal weight parent, children had higher obesity risk if they had an obese father (OR=2.11), an obese mother (OR=7.66), or two obese parents (OR=8.05) [40]. These observations suggested that assortative mating is related to the epidemic of childhood obesity.
Ethnic Differences
Ethnic disparities have been widely discussed as a contributing factor to adiposity. In adults, very large differences between ethnic groups, especially among women, have been observed, with non-Hispanic black individuals exhibiting the highest prevalence of obesity [41]. Asian Indian individuals were observed to have significantly greater total abdominal fat and intra-abdominal adipose tissue and truncal subcutaneous (SC) adipose tissue than Caucasians [42]. Similarly in children, South Asian Indians reportedly had smaller body mass index and waist circumference than Caucasians, and yet both boys and girls from India had higher percent body fat [43]. Hispanic Americans exhibit the greatest trunk, intra-abdominal and SC adipose tissue, followed by European Americans and African American [44•].
In comparison with Caucasian children of the same age, Asian children have lower BMIs by 3-6 units for a given percentage of body fat [45]. Ethnic differences in adiposity had been reported in adolescence, with greater central adiposity being observed in women of Asian ancestry compared with Caucasians [46]. For a given BMI, children and infants of South Asian origin have higher adiposity compared with White Europeans, suggesting that either genetic factors or exposure to maternal physiology contribute to obesity rather than behaviors or diet during childhood or later in life [47].
The risk factors for obesity reportedly exist from as early as the prenatal and early childhood periods [48]. Taveras et al. [49] further suggested that racial or ethnic differences in childhood obesity may be determined by several factors that operate during pregnancy, infancy and early childhood. Compared to Caucasian children, children of Black and Hispanic ancestry had higher odds of rapid infant weight gain, and exhibited lower birth weights for gestational age but had higher BMI z-scores, and a greater prevalence of obesity at the age of three years [49].
Amongst ethnic groups, cultural differences are believed to be one of the contributing factors to the disparities in childhood obesity [50]. For instance, perception on body image occurs in a cultural context and differs by ethnic groups. Women who traditionally feed, educate and take care of their children, also possess their own beliefs about body image, which in turn will have implications toward their children’s own body image [50]. In addition, cultural activities may also be considered as a non-modifiable factor in determining a child’s adiposity. A recent prospective study [51] found negative association between participation in cultural activities with z-scores of waist circumference (WC) and waist-to-height ratio (WHR) in girls; and concluded that cultural activities had a moderating effect on the obesity-susceptibility genes. Although environmental factors pertaining to health-related behaviors or lifestyles and economic disadvantage could contribute to some of the ethnic differences in the prevalence of diseases associated with obesity, these factors cannot explain all the differences in expression and disease patterns due to race or ethnicity. Hence, it is likely that genetics or molecular factors also contribute to the racial disparities in obesity-related comorbidities [52].
Gestational Weight and Intrauterine Factors
The intrauterine environment plays a crucial role in the development of obesity, type 2 diabetes mellitus and metabolic syndrome in the offspring [53, 54]. Shankar and colleagues [55] illustrated that children exposed to maternal obesity in utero were more susceptible to obesity, regardless of birth weight, which seems to indicate that subtle programming of obesity occurs in the absence of clear changes in birth weight. Children of overweight or obese mothers also had more likelihood of being born large for gestational age (LGA) [56•]. These children are at an increased risk of obesity when they also exhibit a high birth weight of more than 4 kg [57].
Pre-pregnancy obesity is the strongest risk factor for childhood obesity [58] and metabolic dysregulation [59]. Higher gestational weight gain (GWG) has been associated with both a greater incidence of pre-eclampsia [60] and a significantly higher risk of gestational diabetes mellitus (GDM) with maternal hyperglycemia as well as fetal hyperglycemia, which can consequently lead to excess fetal insulin and thus fetal overgrowth [61]. Hull and colleagues demonstrated direct relationship between weight gain during pregnancy and birth weight or infant adiposity [62]. Longitudinal data have revealed a strong relationship between gestational weight gain and childhood weight status, regardless of the mother’s pre-pregnancy weight [63] and also independent of genetic factors [64]. It has been suggested that weight status during early human development, especially in the maternally overweight or obese children, can have on-going effects on adiposity and related chronic diseases [65].
In the in utero environment, epigenetic factors may alter gene expression and predispose the fetus to abnormal physical activity and dietary behaviors later in life by compromising the physiological thresholds for energy balance regulation [66]. Moreover, the offspring’s susceptibility to premature chronic diseases may be influenced by constant exposure to excess energy, hormones and growth factors in utero [54]. Human data regarding the effect of maternal lifestyle on epigenetic modifications are scarce; however, animal-model research has demonstrated that the body composition of the offspring changes with maternal diet and is associated with epigenetic alterations in metabolic control genes [67]. Furthermore, the maternal diet may influence the food preferences and feeding responses in the offspring [68]; and, if nutritionally compromised, may promote adiposity as well as early onset of metabolic impairments in the child [69]. The intake of glucose and lipids, such as triglycerides and non-esterified fatty acids, had also been shown to have positive relationship with fetal growth [70, 71•].
Modifiable Risk Factors of Childhood Obesity
Socio-economic Status (SES)
Living conditions and societal factors have great impact on a child’s weight status. In Sweden, boys living in semi-urban and rural areas have a greater risk of obesity [72]. In many developed countries, lower socioeconomic groups reportedly had the highest levels of overweight and the lowest levels of physical fitness, and adolescent girls were particularly at risk [73]. In most countries, children in urban areas were more likely to be obese than those in rural areas. Contemporary populations of children were found to exhibit higher rates of obesity than do those from the lowest socioeconomic groups in high-income countries [74]. In India, significantly more children of higher SES status were obese and overweight than those from lower SES [75, 76].
Positive energy balance, high energy intake, and low levels of energy expenditure on physical activity are strongly associated with urbanization and economic affluence [77]. SES groups, usually low-SES in industrialized countries and high-SES in developing countries, with greater access to energy-dense diets are at a higher risk of being obese. However, the obesity–SES association varies with gender, age, and country [78••]. The findings from a longitudinal survey suggest that rather than having a major direct causative role, family income may act primarily as a proxy for other unobserved characteristics that determine children’s weight status [79].
Diet
Breast-feeding is known to have a consistent protective effect against obesity in children [80]; however, a quantitative review by Owen et al. concluded that the precise magnitude of association is still unclear [81]. The macronutrient composition and bioactive substances of breast milk may influence metabolic programming and the regulation of body fatness and growth rate [80]. Formula-fed infants had higher insulin levels and lower leptin levels compared with breast-fed infants; and these proteins could stimulate fat deposition and lead to the early development of adipocytes [82]. Direct breastfeeding during early infancy has been related to better appetite regulation later in childhood; whereby a study showed that children who were fed human milk in a bottle during the first three months of life were 67% less likely to have good response to satiety during their preschool years compared to children who were directly breastfed [83]. The effect of breastfeeding on infant growth may be a significant determinant of early life programming for obesity and chronic diseases later in life, especially for the offsprings of women with diabetes [84].
The adoption of industrialized Western society lifestyles, including urbanization, Western foods, increased sedentariness and car ownership, has been related to increased obesity. Rapid and marked socio-economic advancement has brought about considerable changes to the lifestyles of communities, including among children; especially with respect to dietary patterns, such as the high intake of energy-dense food, which has been identified as an important factor in body weight control in adults, as well as in children and adolescents [85]. Moreover, alterations in meal patterns are also evident with more families eating out, skipping meals especially breakfast and relying too much on fast foods, which are known to be high in saturated and trans fats, energy dense, and served in large portion sizes [86–88]. Children who ate all three main meals, namely, breakfast, lunch and dinner, daily were reported to have 63% lower risk of being overweight or obese than those who did not [89]. Another study had also further confirmed the protective role of consuming three meals per day with a lower likelihood of overweight or obesity, but only if breakfast was not skipped [90]. Skipping breakfast has been shown to affect children's appetite but not their energy intake at subsequent meals [91•]. When children did not consume breakfast, they were hungrier, less full, and could consume more food prior to lunch; hence, increasing daily dietary intake [92].
The rapid development of fast-food outlets and the easy availability of junk food is also a matter of concern. Children with working parents who are cared for by care providers are more likely to receive food that is high in energy and of poor nutritional value, perhaps because care providers are more concerned with placating their wards than with the long-term health of the children [93]. Furthermore, parents who work outside of the home may also serve more high-calorie pre-prepared, convenience, or fast foods due to time constraints. Additionally, unsupervised children tended to make poorer nutritional choices when preparing their own snacks [94]. It has also been reported that children consumed more fast food items and carbonated drinks as compared to fruits and vegetables, as these food items were easily available through vending machines and school canteens [91•].
Changes in lifestyle, dietary habits, and food marketing have brought about undesirable effects, with large proportions of the population afflicted with various non-communicable diseases associated with over-nutrition, including obesity [95–97]. Available evidence suggests that high-energy intake in early infancy and high consumption of energy-dense foods and sweetened drinks during childhood is associated with increased adiposity [85].
Food marketing to children has been proposed as a means for addressing the global crisis of childhood obesity in recent years. The commercial advertising and marketing of food and beverages influences the diet and health of children and youths. An estimated more than $10 billion a year is spent on marketing of all types of food and beverages to children and youths in America [98]. There is evidence that food marketing to children is (a) massive; (b) expanding in number of avenues, such as product placements, video games, the internet, and cell phones; (c) composed of messages that are almost entirely for nutrient-poor, calorie-dense foods; (d) having harmful effects; and (e) increasing globally and thus difficult to regulate by individual countries [99]. Recently, WHO has produced a set of recommendations to help member countries to either develop or strengthen policy related to marketing of foods and non-alcoholic beverages to children [100].
More recently, vitamin D deficiency has been identified as a new global public health issue. Vitamin D has been suggested to be a potential factor in the prevention of many illnesses, including obesity [101]. Obese individuals were observed to have lower concentrations of 25-hydroxy vitamin D, suggesting that obese individuals may have altered vitamin D and parathyroid hormone physiology [102].
Physical Activity
Physical activity has long been recognized as one of the important determinants of obesity [103] and as a promoter of lifelong positive health behavior in children [104]. The importance of physical activity lies in the basic concept of the Law of Thermodynamics, from which can be derived the fact that human energy expenditure consists of three components; namely, energy that is expended for thermogenesis, energy that is expended for basal metabolism or the basal metabolic rate, and energy that is expended on physical activity [105]. Of these, physical activity is the only factor that can be modified to prevent obesity.
It has been reported that only a third of overweight and obese children engaged in a minimum of 60 minutes of physical activity daily, which suggests that the younger generation led sedentary lifestyles [106]. School children, especially those in Asian countries, have been reported to focus more on academics and are less involved in sports and physical activities [107]. Children also spent much of their leisure time engaged in sedentary activities, such as watching TV or playing computer/video games [108]. Studies have found that normal BMI values were distributed in the higher tertiles of physical activity [109, 110].
A systematic review by Te Velde et al. [111] confirmed that there is an inverse association between total physical activity and overweight; but not, with respect to specific sub-behaviors, such as moderate to vigorous physical activity, aerobic exercise and leisure activity. Jimenez-Pavon et al. also presented evidence that supports negative association between objectively measured physical activity and adiposity, and reported that higher levels of habitual physical activity are protective against child and adolescent obesity [112].
Watching television decreases the amount of time spent on active physical activities [113], and has been associated with increased food consumption either during television viewing or as an indirect result of food advertisements [114]. A study involving students aged 11 to 13 years in California concluded that time spent watching television was significantly associated with obesity [113]. Te Velde et al. [111] also reported moderate evidence for a significant positive association between TV/video/computer screen time and overweight. Increased physical activity and decreased screen-based sedentary behavior were associated with decreased body fat percentages but not decreased BMI [115].
Although the benefits of physical activity have been proven in many studies [103, 104, 111–113], there are a few studies that reported contradicting results regarding physical activity and obesity. A longitudinal study in England found that physical inactivity is the result rather than the cause of obesity, and argued that inactivity does not lead to fatness; hence, explaining why physical activity interventions sometimes fail to prevent excess weight gain in children [116]. Another intervention program conducted among preschool children, reported that physical activity helped to improve motor skills but failed to reduce body mass index [117].
Sleep
Sleep plays an important role in the health of children and adolescents as it allows for the normal diurnal rhythm of hormones that are related to growth, maturation, and energy homeostasis [118•]. Children who sleep for shorter durations have been postulated to have lower energy intake and expenditure; since sleep deprivation is known to lead to alterations in the structure of sleep stage, and hence, giving rise to fatigue, daytime sleepiness, somatic and cognitive problems and low activity levels [119]. Several studies have reported that habitual sleep length is prospectively and independently associated with obesity and mortality [120, 121] with those who sleep for short durations being more likely to be obese [122]. On the other hand, longer sleep duration may reduce the opportunity to eat, and thus prevent over-eating among children and adolescents [123].
Sleep deprivation influences the development of obesity through several possible biological pathways. These include increased sympathetic activity, decreased leptin and growth hormone, elevated cortisol and ghrelin levels, and impaired glucose tolerance [124]. Hormonal alterations may contribute to the selection of energy-dense food, excessive energy intake, changes in energy expenditure, insulin resistance, alterations in the basal metabolic rate, modifications in the thermic effect of food and non-exercise activity thermogenesis [123, 125]. As short sleep duration has been clearly associated with increased risk of childhood obesity, sleep could be a vital factor that needs to be considered in childhood obesity prevention [125].
Visceral adiposity is a significant predictor of obstructive sleep apnea (OSA), with the severity being independent of BMI among obese children [126]. This is supported by another study in which obese children exhibited no difference in head, neck and abdominal subcutaneous fat but instead exhibited more abdominal visceral fat [127]. A longitudinal study revealed that children who slept less were more likely to be overweight and had high body fat values [128•]. Besides, sleep duration decreases with age whereas higher body fat mass and BMI both tend to increase with age [129].
Based on a meta-analysis, the recommended sleep durations are 11 hours or more for children aged below 5 years, 10 hours or more for children aged between 5 and 10 years, and 9 hours or more for children aged 10 years and above [125]. Independent of other risk factors, increasing sleep duration may decrease the prevalence of childhood obesity [130]. The current available evidence is unable to support claims of a secular trend [131]. Future randomized intervention trials are necessary to determine the effectiveness of sleep extension for the prevention of obesity among children and adolescents [130].
Parental Determinants
Parental work schedule [132], parental BMI [133], and maternal smoking habits [134] appear to be important in determining children’s health status, particularly with respect to a healthy body weight. Maternal smoking during pregnancy may be a risk factor for childhood obesity, along with low birth weight [135]. A possible explanation for this may be the impact of catch-up growth in the first year of life on childhood obesity [136]. Children whose mothers smoked during pregnancy were at increased risk for overweight at the ages of 3 to 33 years [134]. Although the mechanisms by which maternal smoking influences the weight of the child are not well characterized, they are possibly due to nicotine, which is transported across the placenta, and carbon monoxide, which potentially influences placental vascular function and may cause fetal hypoxia [134]. Nicotine acts to reduce appetite [137] and body weight, while withdrawal results in hyperphagia and weight gain [138]. It has been suggested that the rapid weight gain of the infant during the early postnatal period may be due to the effect of nicotine withdrawal, similar to the increased craving for food [139].
A positive association has also been observed between childhood obesity and parental BMI. Excessive gains in parental BMI during youth and later life were found to be associated with higher BMI and risk of obesity in the offspring [133]. Similarly, child overweight/obesity was also significantly associated with maternal work hours and paternal non-standard work schedules [132, 140]. However, further research is required to determine the manner in which non-standard work schedules disrupt family life, the quality of children’s diet and their opportunity to participate in physical activity [141].
Conclusion
Obesity is considered to be an epidemic given that its prevalence and severity in both adults and children is rising at alarming rates. This increase is related to the interactions between genetic makeup, intrauterine factors, and environmental living conditions. Obesity may result from genetic susceptibility to a single gene or due to the interactions of multiple genes that influence appetite regulation, adipocyte growth and energy expenditure. Ethnic disparities also contribute to childhood adiposity. Moreover, children from low socioeconomic families in industrialized countries and high socioeconomic background in developing countries are at a higher risk of being obese due to easy accessibility of energy-dense food. Maternal weight status prior to or during gestation may also be a key factor related to the short- and long-term risks of childhood obesity. Furthermore, other parental determinants, such as maternal smoking and lengthy work duration, may worsen the risks of childhood obesity. Contemporary lifestyle changes are also important influences leading to childhood obesity; these changes include increasingly sedentary activities, unhealthy dietary habits and lower quality of sleep. Therefore, deeper understanding of the multi-factorial contributors to obesity is of utmost importance in order to aid in the development of effective interventions aimed at reducing the rates of occurrence of global obesity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Helba M, Binkovitz LA. Pediatric body composition analysis with dual energy X-ray absorptiometry. Pediatr Radiol. 2009;39(7):647–56.
International Food Information Council Foundation: Moving to prevent childhood obesity. Available at www.foodinsight.org/Newsletter/Detail.aspx?topic=Moving_to_Prevent_Childhood_Obesity. Accessed October 2012.
Gahagan S. Child and adolescent obesity. Current Problems in Pediatric and Adolescent Health Care. 2004;34:6–43.
Quah YV, Poh BK, Ismail MN. Metabolic syndrome based on IDF criteria in a sample of normal weight and obese school children. Malaysian J Nutr. 2010;16(2):207–17.
Wee BS, Poh BK, Bulgiba A, et al. Risk of metabolic syndrome among children living in metropolitan Kuala Lumpur: a case control study. BMC Publ Health. 2011;11:333.
World Health Organization: Global strategy on diet, physical activity and health: Childhood overweight and obesity. Available at http://www.who.int/dietphysical activity/children/en/. Accessed October 2012.
Wang Y, Lobstein T. World wide trend in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25.
Budd GM, Hayman LL, Faan RN. Childhood obesity, determinants, prevention and treatment. J Cardiovas Nurs. 2006;21(6):437–41.
Anderson PM, Butcher KF, Levine PB. Economic perspectives on childhood obesity. Econ Perspectives. 2003;27(3):30–48.
Mountjoy K. Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochem J. 2010;428:305–24.
Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96(1):E181–8.
Hsuchou H, Kastin AJ, Wu X, et al. Corticotropin-releasing hormone receptor-1 in cerebral microvessels changes during development and influences urocortin transport across the blood-brain barrier. Endocrinology. 2010;151(3):1221–7.
Leshan RL, Opland DM, Louis GW, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine-and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30(16):5713–23.
Davis JF, Choi DL, Schurdak JD, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiat. 2011;69(7):668–74.
Byerly MS, Simon J, Lebihan-Duval E, et al. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am J Physiol-Reg, I. 2009;296(4):R1180–9.
Tolson KP, Gemelli T, Gautron L, et al. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30(10):3803–12.
Farooqi IS. Genetic, molecular and physiological insights into human obesity. Eur J Clin Invest. 2011;41(4):451–5.
Rankinen T, Bray MS, Hagberg JM, et al. The human gene map for performance and health-related fitness phenotypes: the 2005 update. Med Sci Sports Exercise. 2006;38(11):1863–88.
Saunders CL, Chiodini BD, Sham P, et al. Meta-Analysis of Genome-wide Linkage Studies in BMI and Obesity. Obesity. 2012;15(9):2263–75.
•• Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. This paper continued to identify the BMI-associated loci, which consisted of 14 known loci from previous GWA studies with additional 18 loci from large Europe children. The authors were also examined the association of the BMI loci with metabolic traits and the possible function in body weight regulation.
Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Wardle J, Carnell S, Haworth CM, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93(9):3640–3.
Speakman JR. A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metabolism. 2007;6(1):5–12.
Bradfield JP, Taal HR, Timpson NJ, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44(5):526–31.
Scherag A, Dina C, Hinney A, et al. Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet. 2010;6(4):e1000916.
Fernandez JR, Klimentidis YC, Dulin-Keita A, Casazza K. Genetic influences in childhood obesity: recent progress and recommendations for experimental designs. Int J Obes. 2012;36:479–84.
Faith MS, Pietrobelli A, Heo M, et al. A twin study of self-regulatory eating in early childhood: estimates of genetic and environement influences, and measurement considerations. Int J Obes. 2012;36:931–7.
Agfhani A, Goran MI. Racial differerences in the associatin of subcutaneous and visceral fat on bone mineral content in prepubertal children. Calcif Tissue Int. 2006;79:383–8.
Lee S, Kuk JL, Hannon TS, Arslaian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity. 2008;16:1066–71.
Liu A, Byrne NM, Kagawa M, et al. Ethnic differences in body fat distribution among Asian pre-pubertal children: a cross-sectional multicenter study. BMC Publ Health. 2011;11:500.
Silventoinen K, Rokholm B, Kaprio J, Sørensen TIA. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes. 2010;34:29–40.
Manco M, Dallapiccola B. Genetics of Pediatric Obesity. Pediatrics. 2012;130(1):123–33.
Burrage LC, McCandless SE. Genetics of childhood obesity. US Pediatrics Rev. 2007;1:60–3.
Bauer F, Elbers CC, Adan RA, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90(4):951–9.
Phillips CM, Kesse-Guyot E, McManus R, et al. High Dietary Saturated Fat Intake Accentuates Obesity Risk Associated with the Fat Mass and Obesity–Associated Gene in Adults. J Nutr. 2012;142(5):824–31.
Bouchard C. Childhood obesity: are genetic differences involved? Am J Clin Nutr. 2009;85(5):1494s–501.
Di Castelnuovo A, Quacquaruccio G, Donati MB, et al. Spousal concordance for major coronary risk factors: a systematic review and meta-analysis. Am J Epidemiol. 2009;169(1):1–8.
Power C, Pouliou T, Li L, et al. Parental and offspring adiposity associations: insights from the 1958 British birth cohort. Annal Hum Biol. 2011;38(4):390–9.
Jeffery RW, Rick AM. Cross-Sectional and Longitudinal Associations between Body Mass Index and Marriage-Related Factors. Obesity Res. 2012;10(8):809–15.
Ochoa MC, Azcona C, Moreno-Aliaga MJ, et al. Influence of parental body mass index on offspring body mass index in a Spanish population. Revista Espanola de Obesidad. 2009;7(6):395–401.
Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28.
Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int JObes. 2010;35(2):167–87.
Lakshmi S, Metcalf B, Joglekar C, et al. Differences in body composition and metabolic status between white UK and Asian Indian children (EarlyBird 24 and the Pune Maternal Nutrition Study). Ped Obes. 2012;7:347–54.
• Casazza KL, Hanks J, Beasley TM, et al. Beyond thriftiness: independent and interactive effects of genetic and dietary factors on variations in fat deposition and distribution across populations. Am J Phys Anthropol. 2011;145(2):181–91. This paper described the interactive contribution of genetic and diet in body fat storage of children from different population. For instance, adiposity measures were associated with European, particularly boys compare to African and girls at their counterparts.
Liu A, Byrne NM, Kagawa M, et al. Ethnic differences in the relationship between body mass index and percentage body fat among Asian children from different background. Brit J Nutr. 2011;106:1390–7.
Morimoto Y, Maskarinec G, Conroy SM, et al. Asian ethnicity is associated with a higher trunk/peripheral fat ratio in women and adolescent girls. J Epidemiol. 2012;22(2):130–5.
Stanfield KM, Wells JC, Fewtrell MS, et al. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol. 2012;41(5):1409–18.
Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357.
Taveras EM, Gillman MW, Kleinman K, et al. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics. 2010;125(4):686–95.
Caprio S, Daniels SR, Drewnowski A, et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment. Diabetes Care. 2008;31(11):2211–21.
Cuypers K, Ridder KD, Kvaløy K, et al. Leisure time activities I adolescence in the presence of susceptibility genes for obesity: risk or resilience against overweight in adulthood? The HUNT study. BMC Publ Health. 2012;12:820.
Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89(6):2590–4.
Huang RC, Burke V, Newnham J, et al. Perinatal and childhood origins of cardiovascular disease. Int J Obes. 2006;31(2):236–44.
Gluckman PD, Hanson MA, Hanson CX, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England J Med. 2008;359(1):61–73.
Shankar K, Harrell A, Liu X, et al. Maternal obesity at conception programs obesity in the offspring. Am J Physiol-Reg I. 2008;294(2):R528–38.
• Ferraro ZM, Barrowman N, Prud'homme D, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Maternal-Fetal Neonatal Med. 2012;25(5):538–42. This paper found out that higher pre-pregnancy BMI and gestational weight gain was associated with increased rate of large-for-gestational-age birth weight after controlling for maternal and gestational age, pre-pregnancy weight, parity and smoking.
Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obesity Rev. 2011;12(7):525–42.
Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. International J Obes. 2009;33(7):743–52.
Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–74.
Langer O, Yogev Y, Xenakis EMJ, Brustman L. Overweight and obese in gestational diabetes: The impact on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1768–76.
Hull HR, Thornton JC, Ji Y, et al.: Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205(3):211 e1-7.
Schack-Nielsen L, Michaelsen KF, Gamborg M, et al. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes. 2010;34(1):67–74.
Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376(9745):984–90.
Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9(4):1263–307.
McMillen IC, Rattanatray L, Duffield JA, et al.: The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;71-81.
Levin BE. Epigenetic influences on food intake and physical activity level: review of animal studies. Obesity. 2008;16 Suppl 3:S51–4.
Bayol SA, Farrington SJ, Stickland NCA. Maternal 'junk food' diet in pregnancy and lactation promotes an exacerbated taste for 'junk food' and a greater propensity for obesity in rat offspring. Brit J Nutr. 2007;98(4):843–51.
Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a 'junk food' diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586(13):3219–30.
Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31(9):1858–63.
• Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204(6):479–87. This review article gave an understanding of the metabolic environment of obese diabetic women and lipid metabolism affecting fetal adiposity through perinatal metabolic programming since these issues relates to the increasing trends of obesity.
Moraeus L, Lissner L, Yngve A, et al. Multi-level influences on childhood obesity in Sweden: societal factors, parental determinants and child’s lifestyle. Int J Obes. 2012;36(7):969–76.
Dollman J, Norton K, Norton L. Evidence for secular trends in children’s physical activity behaviour. Br J Sports Med. 2005;39:892–7.
Han JC, Lowlor DA, Kimm SYS. Childhood obesity-2010: progress and challenges. Lancet. 2010;375(9727):1737–48.
Chhatwal J, Verma M, Riar SK. Obesity among pre-adolescent and adolescents of a developing country (India). Asia Pacific J Clin Nutr. 2004;13(3):231.
Manios Y, Panagiotakos DB, Pitsavos CE, et al. Implication of socio-economic status on the prevalence of overweight and obesity in Greek adults: the ATTICA study. Health policy (Amsterdam, Netherlands). 2005;74(2):224.
Wells JCK, Marphatia AA, Cole TJ, McCoy D. Associations of economic and gender inequality with global obesity prevalence: understanding the female excess. Social Sci Med. 2012;75:482–90.
•• Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psych. 2012;24(3):176–88. This paper not only gave an overview of current prevalence of childhood obesity and its association with socio-economic status, but also foreseen the time trends of childhood obesity.
Chia Y. Dollars and pounds: the impact of family income on childhood weight. Appl Econ. 2012;45(14):1931–41.
Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity–a systematic review. Int J Obes. 2004;28(10):1247–56.
Owen CG, Martin RM, Whincup PH, et al. Effect of Infant Feeding on the Risk of Obesity Across the Life Course: A Quantitative Review of Published Evidence. Pediatrics. 2005;115(5):1367–77.
Savino F, Nanni G, Maccario S, et al. Breast-fed infants have higher leptin values than formula-fed infants in the first four months of life. J Pedia Endocrinology and Metabol. 2004;17(11):1527–32.
Disantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phy. 2011;8:89.
Crume TL, Ogden L, Maligie M, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34(3):641–5.
Pérez-Escamilla R, Obbagy JE, Altman JM, et al. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Dietetic. 2012;112(15):671–84.
Fulkerson JA, Neumark-Sztainer D, Hannan PJ, Story M. Family meal frequency and weight status among adolescents: cross-sectional and 5-year longitudinal associations. Obesity. 2008;16(11):2529–34.
Rosenheck R. Fast food consumption and increased caloric intake: a systematic review of a trajectory towards weight gain and obesity risk. Obesity Rev. 2008;9(6):535–47.
Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr. 2011;141(7):1381–9.
Eloranta A, Lindi V, Schwab U, et al. Dietary factors associated with overweight and body adiposity in Finnish children aged 6–8 years: the PANIC Study. Int J Obes. 2012;36(7):950–5.
Antonogeorgos G, Panagiotakos D, Papadimitriou A, et al. Breakfast consumption and meal frequency interaction with childhood obesity. Ped Obes. 2012;1:65–72.
• Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr. 2011;93(2):284–91. This study gave an insight that over-compensation for the missing calories from skipping breakfast by eating more among children despite differences in subjective feelings of hunger and appetite.
Kral TVE, Allison DB, Birch LL, et al. Caloric compensation and eating in the absence of hunger in 5-to 12-y-old weight-discordant siblings. Am J Clin Nutr. 2012;96(3):574–83.
Vasquez F, Salazar G, Andrade M, et al. Energy balance and physical activity in obese children attending day-care centres. Eur J Clin Nutr. 2006;60(9):1115–21.
van der Horst K, Oenema A, Ferreira I, et al. A systematic review of environmental correlates of obesity-related dietary behaviors in youth. Health Edu Res. 2007;22(2):203–26.
Gillis LJ, Bar-Or O. Food away from home, sugar-sweetened drink consumption and juvenile obesity. J Am Coll Nutr. 2003;22(6):539–45.
Amin TT, Al-Sultan AI, Ali A. Overweight and obesity and their relation to dietary habits and socio-demographic characteristics among male primary school children in Al-Hassa, Kingdom of Saudi Arabia. Eur J Nutr. 2008;47(6):310–8.
Neumark-Sztainer D, Story M, Hannan PJ, Croll J. Overweight status and eating patterns among adolescents: where do youths stand in comparison with the healthy people 2010 objectives? Am J Public Health. 2002;92(5):844–51.
McGinnis JM, Gootman JA & Kraak VI: Food marketing to children and youth: threat or opportunity? United States of America: National Academic Press; 2006.
Harris JL, Pomeranz JL, Lobstein T, Brownell KD. A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Ann Rev Public Health. 2009;30:211–25.
WHO: A framework for implementing the set of recommendations on the marketing of foods and non-alcoholic beverages to children. Geneva, Switzerland. 2012.
Holick MF. Vitamin D deficiency. New England J Med. 2007;357(3):266–81.
Earthman C, Beckman L, Masodkar K, Sibley S. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes. 2011;36(3):387–96.
Bammann K, Sioen I, Huybrechts I, et al. The IDEFICS validation study on field methods for assessing physical activity and body composition in children: design and data collection. Int J Obes. 2011;35:S79–87.
Lazaar N, Aucouturier J, Ratel S, et al. Effect of physical activity intervention on body composition in young children: influence of body mass index status and gender. Acta Paediatrica. 2007;96(9):1321–5.
Dulloo AG. Energy balance and body weight homeostasis. In: Kopelman PG, Caterson ID, Dietz WH, editors. Clinical Obesity in Adults and Children. Third Edition. Oxford: Blackwell; 2010. p. 67–81.
Pate RR, Freedson PS, Sallis JP, et al. Compliance with physical activity guidelines: Prevalence in a population of children and youth. Ann Epidemiol. 2002;12(5):303–8.
Food and Nutrition Research Institute: Physical activity: Do Asian children get enough? Available at http://www.fnri.dost.gov.ph/index.php?option=content&task=view&id=704
Merchant AT, Dehghan M, Behnke-Cook D, Anand SS. Diet, physical activity and adiposity in children in poor and rich neighbourhoods: a cross sectional comparison. Nutr J. 2007;6(1):1.
Patrick K, Norman GJ, Calfas KJ, et al. Diet, physical activity and sedentary behaviors as risk factors for overweight in adolescence. Arch Ped Ado Med. 2004;158(4):385.
Ortega FB, Ruiz JR, Sjöström M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phy Act. 2007;4(1):61.
Te Velde S, Van Nassau F, Uijtdewilligen L, et al. Energy balance-related behaviours associated with overweight and obesity in preschool children: a systematic review of prospective studies. Obes Rev. 2012;13:56–74.
Jimenez-Pavon D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: Systematic review. Int J Ped Obes. 2010;5(1):3–18.
Özmert EN, Özdemir R, Pektas A, et al. Effect of activity and television viewing on BMI z-score in early adolescents in Turkey. World J Pediatr. 2011;7(1):37–40.
Temple JL, Giacomelli AM, Kent KM, Roemmich JN, Epstein LH. Television watching increases motivated responding for food and energy intake in children. Am J Clin Nutr. 2007;85(2):355–61.
Carlson JA, Crespo NC, Sallis JF, et al. Dietary-Related and Physical Activity-Related Predictors of Obesity in Children: A 2-Year Prospective Study. Childhood Obesity (Formerly Obesity and Weight Management). 2012;8(2):110–5.
Metcalf BS, Hosking J, Jeffery AN, et al. Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45). Arch Dis Child. 2011;96:942–7.
Reilly JJ, Kelly L, Montgomery C, et al. Physical activity to prevent obesity in young children: cluster randomised controlled trial. BMJ. 2006;333:1041–3.
• Jiang F, Zhu S, Yan C, et al. Sleep and obesity in preschool children. J Ped. 2009;154(6):814–8. Caregivers who slept less and mothers with higher education related to short sleep duration of pre-school children, especially decreased bedtime sleep hours at night. Children who slept less than <9.4 hours per night were more likely to be obese than who slept ≥11 hours (odds ratio [OR], 4.76; 95% CI, 1.28-17.69; P<0.05; OR, 3.42; 95% CI, 1.12-10.46; P<0.05).
Al Mamun A, Lawlor DA, Cramb S, et al. Do childhood sleeping problems predict obesity in young adulthood? Evidence from a prospective birth cohort study. Am J Epidemiol. 2007;166(12):1368–73.
Cappuccio FP, Taggart FM, Kandala NB, Currie A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619.
Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12(4):289–98.
Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol. 2008;77(3):266–76.
Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91(11):881–4.
Eisenmann JC. Insight into the causes of the recent secular trend in pediatric obesity: Common sense does not always prevail for complex, multi-factorial phenotypes. Prevent Med. 2006;42(5):329–35.
Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity. 2008;16(2):265–74.
Canapari CA, Hoppin AG, Kinane TB, et al. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. Journal of clinical sleep medicine: JCSM. 2011;7(3):268–73.
Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Resp Critical Care Med. 2011;183(6):782–7.
• Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712–2. This paper showed that the pre-school children who sleep less gain significantly more fat mass overtime even after adjustment for multiple determinants of body composition during growth. Each additional hour of sleep reduced the adjusted fat mass index by 0.48 (0.10 to 0.86) for the change from age 3 to 7.
Bayer O, Rosario AS, Wabitsch M, Von Kries R. Sleep duration and obesity in children: is the association dependent on age and choice of the outcome parameter? Sleep. 2009;32(9):1183.
Nielsen LS, Danielsen KV, Sørensen TIA. Short sleep duration as a possible case of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12(2):78–92.
Matricciani L, Olds T, Williams M. A review of evidence for the claim that children are sleeping less than in the past. Sleep. 2011;34(5):651.
Morrissey TW, Dunifon RE, Kalil A. Maternal employment, work schedules, ad children’s body mass index. Child Dev. 2011;82:66–81.
Li L, Law C, Conte RL, Power C. Intergenerational influences on childhood body mass index: the effect of parental body mass index trajectories. Am J Clin Nutr. 2009;89(2):551–7.
Oken E, Levitan E, Gillman M. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes. 2007;32(2):201–10.
Ong KKL, Preece MA, Emmett PM, Emmett ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Ped Res. 2002;52(6):863–7.
Von Kries R. Maternal Smoking during Pregnancy and Childhood Obesity. Am J Epidemiol. 2002;156(10):954–61.
Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53(4):618–32.
Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Therap. 2011;90(1):164–8. doi:164.
Lerman C, Berrettini W, Pinto A, et al. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology. 2004;174(4):571–7.
Mindlin M, Jenkins R, Law C. Maternal employment and indicators of child health: a systematic review in pre-school children in OECD countries. J Epidemiol Comm Health. 2009;63:340–50.
Champion SL, Rumbold AR, Steele EJ, et al. Parental work schedules and child overweight and obesity. Int J Obes. 2012;36:573–80.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ang, Y.N., Wee, B.S., Poh, B.K. et al. Multifactorial Influences of Childhood Obesity. Curr Obes Rep 2, 10–22 (2013). https://doi.org/10.1007/s13679-012-0042-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-012-0042-7