Abstract
The prevalence of overweight and obesity has increased worldwide to epidemic proportions. Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis and chronic stress exposure are hypothesized to contribute to obesity development. In this review, we discuss the potential role of the HPA axis for energy balance regulation, with particular attention to energy intake. We present evidence from human and animal studies that highlight the bidirectional relationship between HPA axis functioning and energy intake. Of particular interest is the association between dysregulation of the HPA axis and altered homeostatic and non-homeostatic food intake regulation. Lastly, we discuss a model depicting a role for a hyperactive HPA axis in overeating and the development of obesity, suggesting chronic stress as a major risk factor for excessive weight gain and (visceral) obesity.
Similar content being viewed by others
Introduction
The prevalence of overweight and obesity has increased worldwide to epidemic proportions [1]. Obesity results from a chronic dysregulation of energy balance, with energy intake exceeding energy expenditure, leading to the storage of excessive energy as fat [2]. Elements of modern society, including western diet, sedentary lifestyle, and stress may contribute to a positive energy balance and the development of obesity, possibly through dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis is one of the neuro-endocrine axes that plays an important role in the regulation of the stress-response, by regulating the secretion of glucocorticoids: cortisol in humans and corticosterone in animals [3]. The cascade of the HPA axis beholds that first the hypothalamus produces and releases corticotropin-releasing hormone (CRH), which subsequently stimulates the synthesis and release of adrenocorticotropin (ATCH) from the anterior pituitary. ACTH is produced from a larger precursor protein namely the proopiomelanocortin (POMC) protein, and stimulates the synthesis and release of cortisol or corticosterone by the adrenal cortex. Physiological glucocorticoid levels follow a circadian rhythm; an early morning peak just prior to awakening, a rapid decrease over the next few hours, and then a more gradual decline over the course of the day, to very low levels at bedtime [4].
Evidence for the involvement of the HPA axis in the regulation of body weight and body fat distribution is found in two extremes of plasma cortisol concentrations in humans; Addison’s disease (hypocortisolism) that is related to weight loss, and Cushing’s syndrome (hypercortisolism) that is related to rapid weight gain, particularly fat gain in trunk and face but not in the limbs [5]. Additional support is found in obese subjects with visceral fat accumulation, who show chronic hyper-activation of the HPA axis, namely decreased salivary and serum cortisol levels, increased urinary secretion of cortisol, enhanced awakening cortisol response, as well as increased cortisol secretion after physical and psychological stressors [6–13]. In our latest study, we observed an inverse relationship between HPA axis functioning and visceral fat accumulation after standardized, high intensity, physical activity accompanied by ingestion of 4 mg dexamethasone [14]. This suggests that visceral fat accumulation relates to disturbance of HPA axis functioning under basal and challenged conditions.
The literature describes several possible mechanisms through which the HPA axis may contribute to the development of obesity. With respect to the effect of HPA axis functioning on energy expenditure we earlier reported dual effects. We argued that the stimulatory actions of glucocorticoids on energy expenditure are overruled by other, inhibitory actions during prolonged or chronic exposure [15]. A recent study by Westerterp et al. indicated that energy expenditure did not change over the last few decades [16]. This suggests that the disturbance in energy balance is mainly caused by increased energy intake. Therefore, the focus of the present review will be on the relationship of HPA axis activity with energy intake. We start by describing the interactions between HPA axis functioning and energy intake regulation. Followed by the description of the homeostatic and non-homeostatic pathways through which the HPA axis may affect energy intake, and ultimately body weight. This will lead to a proposed model on the relationship between alterations in HPA axis functioning, energy intake regulation, and (visceral) obesity development.
HPA Axis Functioning and Energy Intake
Stress appears to alter overall energy intake in two ways; under- or overeating, a fact which seems to be influenced by the nature of the stressor, stressor severity and individual predisposition [17]. A retrospective survey of United States Marine’s food intake during combat showed that live threatening stress resulted in a decrease in energy intake. While a study in a student population showed that examination stress resulted in an increase in energy intake [17]. In concordance with animal studies, severe stress appears to decrease energy intake, while milder forms of stress increases energy intake [17]. A recent review by Scott et al. elegantly describes the role of psychosocial stress in the development of obesity, in particular the role of social stress, which is found in the workplace in humans and in subordination models in animals [18]. Additionally, more recently, one type of stress has gained a lot of attention in the scientific community, namely sleep deprivation. Total or partial sleep deprivation is associated with increased cortisol secretion, and subsequent increased energy intake [19, 20]. With the average amount of sleep in the general population decreasing, sleep deprivation may become one of the most important moderate stressors present in our society, next to work related and emotional stressors.
With regard to the direct orexigenic effect of glucocorticoids on energy intake, animal studies provide us with some convincing evidence; they show a clear reversal of the anorectic effects of adrenalectomy through corticosterone replacement [21, 22]. Human studies assessing the effects of cortisol on energy intake are limited, but generally support the findings in laboratory animals [23, 24]. Stimulation of energy intake by glucocorticoids is however macronutrient specific. When rats had a free choice for different chow compositions, corticosterone withdrawal and subsequent replacement principally affected fat intake, which is mediated by an effect of corticosterone on insulin secretion [22]. Moreover, it was shown that rats subjected to repeated stress specifically increased the fat and sugar components of the free choice high fat high sugar diet [25]. This suggests that actions of glucocorticoids may underlie the preference for certain macronutrients and kinds of foods in humans after stress, in particular foods high in saturated fat and sugar [22, 26–28].
The literature however proposes a bidirectional relationship between HPA axis functioning and energy intake. From animal studies it has become clear that the ingestion of high fat/sugary foods decreases HPA axis activation and brain corticotropin-releasing-factor (CRF) [22, 29], which however did result in insulin resistance [30]. In addition, it has been shown that stressed people that overeat have decreased cerebrospinal CRF levels and HPA axis activity [31]. It has therefore been postulated that people eat foods high in fat and sugar in an attempt to reduce the neural network involved in the stress response [32]. Also our laboratory showed particular effects of food intake on HPA axis functioning. In those studies a single macronutrient containing meal was offered to normal weight men, which resulted in increased plasma cortisol levels in response to the carbohydrate condition, compared to the control, protein or fat conditions [33•]. These findings are supported by other, human as well as animal studies; while rats responded with reduced HPA axis activity to a high protein diet [34], female viscerally obese women responded with increased HPA axis activity to a high carbohydrate meal [35]. These studies suggest that the altered HPA axis response to certain macronutrients, may contribute to the vicious cycle of HPA activity and overeating, observed in obesity. Stress can trigger overconsumption of comfort food, high in sugar and fat [27], which further increases HPA axis activity [33•]. This hypothesis might however be too simple, as studies using mixed meals instead of single macronutrients did not detect an effect of macronutrients on HPA axis activity [36], or even came to contradictory conclusions [37, 38]. Studies from our laboratory did not observe effects of consumption of meals with different macronutrient contents (high-protein vs. high-carbohydrate) on cortisol levels or mood when the subjects were stressed [39, 40]. Generally, the use of mixed meals instead of single macronutrients [33•, 34, 35] may limit the detection of possible effects of macronutrients on cortisol concentrations. Another explanation may be gender related differences and gender selection, associated with the experiments. Our latest experiment showed higher HPA axis activity following a meal in men compared to women, irrespective of sex-specific differences in body composition, body fat distribution, psychological variables, or in age [41]. Finally, freedom of choice might influence the relationship, while rats forced to eat high fat and sugary foods did not show decreased HPA axis activation, while those with free choice did [22, 42]. Further research is required to clarify the interaction between energy intake and HPA axis functioning.
Together, these findings demonstrate a close relationship between the HPA axis activation and energy intake regulation. The literature suggests several homeostatic and non-homeostatic mechanisms through which HPA axis functioning may regulate energy intake. These mechanisms will be discussed in the following sections.
HPA Axis and Homeostatic Food Effects on Intake Regulation
Activity of the HPA axis is orchestrated by a complex interplay of glucocorticoids with central intra- and extra-hypothalamic sites [43]. Of particular interest for the interaction of HPA activity and energy intake are the intra-hypothalamic sites, including the paraventricular nucleus (PVN), and the arcuate nucleus (ARC).
HPA Axis, PVN and ARC
Glucocorticoid receptors are abundantly expressed in both, the PVN and in the ARC [44]. The important role of the PVN for the initiation of glucocorticoid secretion is accentuated by experiments showing markedly reduced CRH levels, and stress induced ACTH levels after PVN lesions [45]. In addition to their major role in the stress response, both areas play a co-regulative role for energy intake regulation [46, 47]. Signals representing nutritional status access the PVN directly or indirectly through activation of ARC afferents. The PVN then affects the periphery and stimulates gut hormones release through neuroendocrine output signals including oxytocin, vasopressin and CRH [48]. CRH is considered an anorexigenic signal, as evidenced through studies using direct CRH administration or blockade of the CRH receptors in the PVN [49].
The PVN receives major input from the ARC, where Neuropeptide Y (NPY) and Agouti-related Peptide (AGRP) neurons are co-localized [50]. Both are considered orexigenic peptide hormones. NPY neurons innervate CRH neurons in the PVN and stimulate CRH release. The subsequently stimulated glucocorticoid release then in turn stimulates NPY activity, but decreases hypothalamic CRH, thus forming a positive feedback loop in favour of energy intake stimulation [43]. The interplay between the anorexigenic CRH and the orexigenic NPY neurons is essential in the maintenance of a healthy body weight. Increased NPY concentrations in response to stress may therefore be an underlying biochemical mechanism of stress eating. It should be mentioned that other studies report hyperphagia in the absence of high NPY concentrations [51]. Those however show an increased sensitivity to the orexigenic effect of NPY.
The NPY/AGRP neurons are regulated in part through the leptin and insulin receptors, both of which are abundant in the ARC [52]. NPY/AGRP neurons are inhibited by insulin and leptin signalling and are activated under circumstances of low insulin and leptin concentrations [50, 53]. Through the co-localisation of neurons and receptors, HPA axis signalling affects energy intake regulation directly or indirectly through an effect on the expression of orexigenic and anorexigenic peptides within the ARC. Describing the relationship between HPA axis activity and the signals relevant to homeostatic energy intake regulation demonstrates that the two systems are intricately involved with each other, stronger, even need each other for function [54], but that the balance needed for metabolic health is a rather fragile framework.
HPA Axis and Adiposity Signals: Insulin and Leptin
But glucocorticoid receptors are abundantly expressed in both the PVN and in the ARC [44]. The ARC is an area where insulin and leptin, which circulate in proportion to fat mass, convey signals about energy status. With low energy stores, leptin and insulin concentrations will be low, which removes inhibition within the ARC to stimulate feeding behaviour. Thus, insulin in the brain serves a catabolic purpose through directly inhibiting orexigenic NPY/AGRP neurons and exciting anorexigenic POMC/cocaine- and amphetamine-regulated transcript (CART) neurons in the ARC [55]. Glucocorticoids interfere with insulin signalling on a peripheral and central level. Centrally, glucocorticoid signalling undermines the antagonizing effect of insulin on NPY, thereby stimulating energy intake [56]. In the periphery, steady state concentrations of glucocorticoids are associated with increases in plasma insulin concentrations [22]. Chronically elevated glucocorticoids exert diabetogenic effects not only through hyperinsulinemia, but also by impairing the insulin induced translocation of the intracellular glucose transporter, as well as through interference with receptor binding in liver and skeletal muscle [57•]. There is converging evidence for the co-occurrence of peripheral and central insulin resistance. While the mechanisms are not fully elucidated, chronically elevated levels of glucocorticoids may play a role through their contribution to prolonged hyperinsulinemia, and their interference with insulin reception in the periphery and the brain. The development of glucocorticoid induced insulin resistance may also help explain the sometimes counterintuitive results regarding the effect of glucocorticoids on energy intake. Due to the insulin stimulatory effect, glucocorticoids could be expected to generally decrease energy intake. Removing or reducing insulin action (through either insulin deficiency or insulin resistance) removes the inhibitory effect, and thus glucocorticoids will then stimulate feeding behaviour [22].
Similar to their effect on insulin, glucocorticoids stimulate systemic leptin concentrations [58], while they inhibit the central action of leptin [59]. In the brain, leptin administration increases hypothalamic CRF, likely through the ARC, which contains an abundance of leptin receptors [52]. This in turn decreases peripheral glucocorticoid release from the adrenals through the negative feedback loop. On a peripheral level, the antagonistic relationship between leptin and glucocorticoids was shown through a direct inhibition of corticosteroid production and a reduction in basal cortisol secretion after incubation with recombinant leptin [60]. Similar to insulin glucocorticoids may be implicated in the development of leptin resistance through the stimulation of hyperleptinemia in the periphery.
HPA Axis and Non-homeostatic Effects on Food Intake Regulation
In addition to the homeostatic pathways involved in energy intake regulation, non-homeostatic regulation of energy intake, including processes such as reward perception, and cognition, were shown to be able to overrule homeostatic regulatory mechanisms [61].
HPA Axis and Reward
Recent studies in animals even suggest that exclusively the non-homeostatic properties of energy intake result in dampening HPA axis reactivity [62•, 63]. Evidence for the HPA axis influencing non-homeostatic eating in humans is found in some of our own studies, which use the ‘eating in the absence of hunger’ paradigm. Stress induced eating in the absence of hunger, observed through increased snack intake in stressed subjects, who just ate a regular lunch [64, 65]. Especially in (viscerally) obese participants, changes in food reward perception were involved in stress augmented eating in the absence of hunger [66]. Like addictive drugs, palatable foods may act as their own reinforcer, thereby stimulating their own intake. Berridge and Robinson defined two distinct psychological processes determining the reinforcing value of food, namely “liking” and “wanting”. The combination of liking and wanting, defines the rewarding value of a given item and thereby its specific maximum of perceived food reward [67]. A recent study from our laboratory showed that stress was associated with reduced food liking in both lean and overweight subjects [68]. The processes underlying liking and wanting are regulated by different neural networks, and are mediated by different neurotransmitters, the latter being the opioid, endocannabinoid, serotonergic and dopaminergic systems [69, 70], all of which are modulated by glucocorticoids [71•]. In studies that investigated food reward specifically with various paradigms, using food images, smells and tastes, brain areas involved were the amygdala, striatum, hippocampus anterior cingulate cortex, and orbitofrontal cortex [71•, 72]. Regarding the influence of stress on reward specific brain activation, we observed that the reward signalling-associated regions, such as the amygdala, putamen, hippocampus and cingulate cortex showed lower activation while choosing food in the stress condition compared to the rest condition [65], especially in overweight subjects [73]. In a subsequent study, we showed that in rest, behavioural liking and wanting were supported by task related signalling in the anterior insula, nucleus accumbens and thalamus. When stressed however, task related brain activation representative of behavioural liking and wanting appeared in more reward related regions, particularly post-meal [74]. Together, these results suggest that high HPA-axis activation disrupts and redirects normal liking and wanting related brain signalling [73, 74]. Together with previous studies, our studies proposes stress to decrease the sensitivity of the neural correlates regulating food reward [65, 71•, 73, 74], thereby facilitating stress induced non-homeostatic eating.
HPA Axis and Cognition
In addition to the influence of the HPA axis on reward, relationships between stress and cognition have been observed. Attention and memory are some of the cognitive processes that are affected by stress [75]. Decreased attention to external stimuli and reduced short-term memory was shown during stress in animals and humans [76, 77]. The role of attention and working memory in eating behaviour in humans is a relatively new subject. Studies have shown that distraction humans during a meal (i.e. watching television or reading), not only increases energy intake at that moment, but also later on [78]. This suggests a high risk for overeating, when little attention is paid to the food consumed. The importance of working memory in energy intake regulation has been illustrated by recent studies from Higgs et al [79]. Enhancing the memory of the last meal, decreased later snack intake, while disruption of encoding of the last meal in memory increased subsequent snack intake [79]. Combined, these findings support the hypothesis that stress could decrease attention to food and working memory of energy intake, resulting in increased energy intake in stressed people. Until now no studies have tested this hypothesis, but it may pose a plausible explanation for increased non-homeostatic eating during stress.
The relationship between HPA axis functioning and cognition is bidirectional. Humans show tendencies to cognitively moderate their energy intake, a phenomenon that has been called “dietary restraint”. Specific questionnaires, such as the Three Factor Eating Questionnaire (TFEQ) can be used to assess dietary restraint. In an earlier study, we showed that high levels of dietary restraint are related to hyperactivity of the HPA-axis [80]. Accordingly, three studies reported that salivary cortisol at one time point (time point not specified) and 24-hour cortisol excretion were significantly higher in restrained women compared with unrestrained women [81, 82]. Although it cannot be excluded that high ambient cortisol levels increase cognitive awareness of caloric intake, the opposite (i.e. high level of dietary stress increased HPA axis activity) seems a somewhat more tempting hypothesis. Dietary restraint is positively correlated with body fat percentage [83], and the load of continuously restrained eating behaviour is reportedly perceived as stressful [84]. Still, dietary restraint may be a risk factor for stress-induced hyperphagia, as studies showed that restrained eaters experiencing psychological stress increased their energy intake, while unrestrained eaters decreased energy intake [26, 85].
Interaction Homeostatic and Non-homeostatic Effects on Energy Intake Regulation
It should be noted, however, that homeostatic and non-homeostatic pathways regulating feeding behaviour are not completely separate systems. Contrary, they are intricately involved with each other, and interact on different levels. Particularly the adiposity signals insulin and leptin play roles for both circuits. Leptin was shown to decrease energy intake when injected in the ventral tegmental area (VTA) of rodents, possibly by lowering the firing rate of VTA dopamine neurons [86]. Moreover, leptin has been shown to decrease performance in behavioural paradigms that assess the rewarding properties of food [87]. Besides leptin, the hormone ghrelin also influences both homeostatic and non-homeostatic pathways. While ghrelin is produced by the stomach and acts as an orexigenic factor, it also is a key regulator of reward-based, hedonic eating behaviour, as ghrelin directly targets the VTA to increase food motivation [88]. An elegant review by Diz-Chaves et al. presented the interaction between the HPA axis and ghrelin [89•]. Stress raises ghrelin levels, which may implicate HPA axis activation as a moderator for ghrelin to increase energy intake by increasing food motivation and feelings of hunger.
The Vicious Cycle of HPA-Activity and Overeating
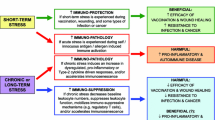
The data presented in this review support the theory proposed by professor Dallman years ago [90]. She proposed that hyperactivity of the HPA axis is part of a vicious cycle between HPA axis activation and energy intake, which makes chronic stress a major risk factor for excessive weight gain and (visceral) obesity (Fig. 1). Susceptibility to chronic stress induced (visceral) obesity can be mediated by genetic variation in the cascade of the HPA axis. Several single nucleotide polymorphisms (SNPs) have been described that cause differences in HPA functioning and/or are involved in obesity development [91]. One of the most often described SNPs is the BclI polymorphism (rs41423247), representing variation in the glucocorticoid receptor. GG carriers of the BclI polymorphism have increased glucocorticoid sensitivity, insulin levels, blood pressure, abdominal visceral fat, and cholesterol levels [92]. In our recent studies, we showed that BclI is primarily related to differences in HPA axis functioning and sensitivity to psychological stress, and not to anthropometric measurements [93, 94]. This suggests that genetic variations cause alterations in HPA axis functioning, which in turn result in anthropometric differences. Overall, these SNP association studies show that humans with certain alleles of the HPA axis SNP genes are more susceptible to chronic stress induced obesity.
Another mediating factor for the vicious cycle between HPA axis activation and energy intake are structural brain abnormalities. This relationship has recently become of interest and a growing line of evidence suggests that alterations in HPA axis functioning are related to a decrease in hippocampal and frontal lobe volume [95]. Similar decreases have been observed in obese and type 2 diabetes patients [96], which suggests a role for altered HPA axis functioning in the relationship between obesity and brain abnormalities [97•]. Recent studies demonstrated that adolescents with insulin resistance had altered HPA axis functioning, smaller hippocampal volumes, and greater frontal lobe atrophy compared to controls [97•]. Mediation analyses indicated pathways whereby altered HPA axis functioning was associated with a higher BMI, which in turn was associated with fasting insulin levels. Those were associated with smaller hippocampal volume and greater frontal lobe atrophy. These findings suggests that HPA axis dysregulation may also impact brain structures through associations with metabolic abnormalities, and strengthen our visceral cycle hypothesis [97•].
Summary and Conclusions
HPA axis activation and chronic stress have several neuronal, metabolic and behavioural consequences, which affect energy intake regulation. All in all, prolonged exposure to elevated glucocorticoid levels and chronic stress may result in a positive energy balance through increased energy intake, without affecting resting energy expenditure. The stimulatory effects of stress on energy intake involve both homeostatic and non-homeostatic pathways. Dysregulation of HPA activation affects homeostatic pathways through to the development of resistance to adiposity signals in the periphery and in the brain. An intact negative feedback loop of the HPA axis is essential for NPY signalling in the brain. Subsequently, disturbed NPY signalling and resistance to adiposity signals may both be players in the vicious cycle of overeating and further HPA axis disturbance.
Non-homeostatic pathways may include neural systems involved in both the “wanting” and “liking” aspects of food reward, cognitive factors such as attention and working memory, as well as the individuals’ attitude towards eating. The non-homeostatic pathways may even be more reinforced through the altered brain systems and brain abnormalities caused by the increased HPA axis activations. Both homeostatic and non-homeostatic pathways result in increased energy intake and altered macronutrient selection, towards more high fat and high sugar foods (Fig. 1). The consequential positive energy balance is likely to result in increased lipogenesis and fat storage, under influence of glucocorticoids. Visceral adipocytes have a fourfold higher number of glucocorticoid receptors than adipocytes in other fat depots. Chronic stress associated hyperactivation of the HPA axis and subsequent hypercortisolism result in increased fat storage particularly in the visceral region, further contributing to the related metabolic adverse consequences. The present review underlines, how the HPA axis play an essential role in the developing obesity epidemic of Western society, where high levels of ambient stress and availability of high fat, sweet foods are abundantly present.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253.
Tremblay A, Perusse L, Bouchard C. Energy balance and body-weight stability: impact of gene-environment interactions. Br J Nutr. 2004;92 Suppl 1:S63–6.
Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–71.
Kurina LM, Weiss LA, Graves SW, Parry R, Williams GH, Abney M, et al. Sex differences in the genetic basis of morning serum cortisol levels: genome-wide screen identifies two novel loci specific to women. J Clin Endocrinol Metab. 2005;90(8):4747–52.
Hankin ME, Theile HM, Steinbeck AW. An evaluation of laboratory tests for the detection and differential diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf). 1977;6(3):185–96.
Travison TG, O'Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf). 2007;67(1):71–7.
Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab. 2008;295(2):E385–92.
Garcia-Prieto MD, Tebar FJ, Nicolas F, Larque E, Zamora S, Garaulet M. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf). 2007;66(2):185–91.
Farag NH, Moore WE, Lovallo WR, Mills PJ, Khandrika S, Eichner JE. Hypothalamic-pituitary-adrenal axis function: relative contributions of perceived stress and obesity in women. J Womens Health (Larchmt). 2008;17(10):1647–55.
Therrien F, Drapeau V, Lalonde J, Lupien SJ, Beaulieu S, Tremblay A, et al. Awakening cortisol response in lean, obese, and reduced obese individuals: effect of gender and fat distribution. Obesity (Silver Spring). 2007;15(2):377–85.
Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13(7):1157–66.
Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77(2):341–6.
Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62(5):623–32.
Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Hypothalamic-pituitary-adrenal (HPA) axis functioning in relation to body fat distribution. Clin Endocrinol (Oxf). 2009;72(6):738–43.
Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94(2):169–77.
Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes (Lond). 2008;32(8):1256–63.
Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–94.
Scott MS, Sakai R. Effects of chronic social stress on obesity. Current Obesity Reports. 2012;In press.
Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9.
Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond). 2012, in press.
la Fleur SE. The effects of glucocorticoids on feeding behavior in rats. Physiol Behav. 2006;89(1):110–4.
la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145(5):2174–85.
Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271(2 Pt 1):E317–25.
Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84(6):1944–8.
Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–62.
Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48(2):195–202.
Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. Food selection changes under stress. Physiol Behav. 2006;87(4):789–93.
Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49.
Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150(5):2325–33.
van Dijk G, Buwalda B. Neurobiology of the metabolic syndrome: an allostatic perspective. Eur J Pharmacol. 2008;585(1):137–46.
Parker G, Roy K, Mitchell P, Wilhelm K, Malhi G, Hadzi-Pavlovic D. Atypical depression: a reappraisal. Am J Psychiatry. 2002;159(9):1470–9.
Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of "comfort food". Proc Natl Acad Sci U S A. 2003;100(20):11696–701.
• Martens MJ, Rutters F, Lemmens SG, Born JM, Westerterp-Plantenga MS. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol Behav. 2010;101(5):563–7. This study suggests that the HPA axis is influenced by energy intake, and that carbohydrate intake alone increases cortisol secretion.
Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D, et al. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R934–42.
Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R. Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes. J Clin Endocrinol Metab. 2002;87(8):3984–8.
Bourrilhon C, Lepers R, Philippe M, Beers PV, Chennaoui M, Drogou C, et al. Influence of protein- versus carbohydrate-enriched feedings on physiological responses during an ultraendurance climbing race. Horm Metab Res. 2010;42(1):31–7.
Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61(2):214–24.
Slag MF, Ahmad M, Gannon MC, Nuttall FQ. Meal stimulation of cortisol secretion: a protein induced effect. Metabolism. 1981;30(11):1104–8.
Lemmens SG, Born JM, Martens EA, Martens MJ, Westerterp-Plantenga MS. Influence of consumption of a high-protein vs. high-carbohydrate meal on the physiological cortisol and psychological mood response in men and women. PLoS One. 2011;6(2):e16826.
Lemmens SG, Martens EA, Born JM, Martens MJ, Westerterp-Plantenga MS. Lack of effect of high-protein vs. high-carbohydrate meal intake on stress-related mood and eating behavior. Nutr J. 2011;10:136.
Martens EA, Lemmens SG, Adam TC, Westerterp-Plantenga MS. Sex differences in HPA axis activity in response to a meal. Physiol Behav. 2012;106(2):272–7.
la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146(5):2193–9.
Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300(1–2):137–46.
Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24(1):269–72.
Makara GB. The relative importance of hypothalamic neurons containing corticotropin-releasing factor or vasopressin in the regulation of adrenocorticotropic hormone secretion. CIBA Found Symp. 1992;168:43–53.
Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–49.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51(2):271–5.
Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring). 2006;14 Suppl 5:259S–65S.
Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611(1):18–24.
Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–2.
Dube MG, Kalra SP, Kalra PS. Low abundance of NPY in the hypothalamus can produce hyperphagia and obesity. Peptides. 2007;28(2):475–9.
Schwartz MW. Biomedicine. Staying slim with insulin in mind. Science. 2000;289(5487):2066–7.
Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44(2):147–51.
Uchoa ET, Silva LE, de Castro M, Antunes-Rodrigues J, Elias LL. Glucocorticoids are required for meal-induced changes in the expression of hypothalamic neuropeptides. Neuropeptides. 2012;46(3):119–24.
Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52(2):232–8.
Sato I, Arima H, Ozaki N, Watanabe M, Goto M, Hayashi M, et al. Insulin inhibits neuropeptide Y gene expression in the arcuate nucleus through GABAergic systems. J Neurosci. 2005;25(38):8657–64.
• Yi CX, Foppen E, Abplanalp W, Gao Y, Alkemade A, la Fleur SE, et al. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes. 2012;61(2):339–45. This study shows for the first time that next to diabetogenic effects through hyperinsulinemia, chronically elevated glucocorticoids also impair insulin induced translocation of the intracellular glucose transporter as well as interference with receptor binding in liver and skeletal muscle.
Laferrere B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. J Clin Endocrinol Metab. 1998;83(10):3742–5.
Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes. 1997;46(4):717–9.
Bornstein SR. Is leptin a stress related peptide? Nat Med. 1997;3(9):937.
Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81(5):781–93.
• Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107(47):20529–34. This study highlights that the palatable/rewarding (i.e. non-homeostatic) properties of sucrose are necessary and sufficient for stress dampening.
Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. 2011;103(1):104–10.
Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring). 2009;17(1):72–7.
Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, et al. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond). 2010;34(1):172–81.
Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS. Stress augments food 'wanting' and energy intake in visceral overweight subjects in the absence of hunger. Physiol Behav. 2011;103(2):157–63.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69.
Martens MJI, Born JM, Lemmens SGT, Adam TC, Westerterp-Plantenga MS. Liking and the orosensory perception of food in a stress vs. rest condition in overweight and normal weight participants. Food Qual Prefer. 2012;26(2):252–8.
Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31(7):1371–81.
Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007;56(5):393–405.
• Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–65. This is an elegant review on the neural networks involved in stress induced eating.
Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–7.
Born JM, Lemmens SG, Martens MJ, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. Am J Clin Nutr. 2011;94(2):392–403.
Born JM, Martens MJ, Rutters F, Lemmens SG, Goebel R, Westerterp-Plantenga MS. High HPA-axis activation disrupts the link between liking and wanting with liking and wanting related brain signaling. Physiol Behav. 2012;105(2):321–4.
Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology. 2000;25(6):535–49.
Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66(1):25–32.
Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: a review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):733–41.
Robinson E. Eating attentively: a systematic review of the effect of food intake memory and awareness on eating. Submitted. 2012.
Higgs S RE, Lee M. Learning and memory processes and their role in eating: implications for limiting food intake in overeaters. Current Obesity Reports. 2012;In press.
Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Hyperactivity of the HPA axis is related to dietary restraint in normal weight women. Physiol Behav. 2009;96(2):315–9.
Anderson DA, Shapiro JR, Lundgren JD, Spataro LE, Frye CA. Self-reported dietary restraint is associated with elevated levels of salivary cortisol. Appetite. 2002;38(1):13–7.
Rideout CA, Linden W, Barr SI. High cognitive dietary restraint is associated with increased cortisol excretion in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2006;61(6):628–33.
de Lauzon-Guillain B, Basdevant A, Romon M, Karlsson J, Borys JM, Charles MA. Is restrained eating a risk factor for weight gain in a general population? Am J Clin Nutr. 2006;83(1):132–8.
Tanofsky-Kraff M, Wilfley DE, Spurrell E. Impact of interpersonal and ego-related stress on restrained eaters. Int J Eat Disord. 2000;27(4):411–8.
Mitchell SL, Epstein LH. Changes in taste and satiety in dietary-restrained women following stress. Physiol Behav. 1996;60(2):495–9.
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10.
Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91(5):473–8.
Perello M, Zigman JM. The Role of Ghrelin in Reward-Based Eating. Biol Psychiatry. 2012, in press.
• Diz-Chaves Y. Ghrelin, appetite regulation, and food reward: interaction with chronic stress. Int J Pept. 2011;2011:898450. This is an elegant review on the role of ghrelin in stress related homeostatic and non-homeostatic energy intake.
Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids–food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633–8.
Derijk RH, de Kloet ER. Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur J Pharmacol. 2008;583(2–3):303–11.
Rosmond R. The glucocorticoid receptor gene and its association to metabolic syndrome. Obes Res. 2002;10(10):1078–86.
Rutters F, Lemmens SG, Born JM, Bouwman F, Nieuwenhuizen AG, Mariman E, et al. Genetic associations with acute stress-related changes in eating in the absence of hunger. Patient Educ Couns. 2010;79(3):367–71.
Rutters F, Nieuwenhuizen AG, Lemmens SG, Bouwman F, Mariman E, Westerterp-Plantenga MS. Associations between anthropometrical measurements, body composition, single-nucleotide polymorphisms of the hypothalamus/pituitary/adrenal (HPA) axis and HPA axis functioning. Clin Endocrinol (Oxf). 2011;74(6):679–86.
Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–90.
Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese adolescents with Type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neurosci Med. 2011;2(1):34–42.
• Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology. 2012;37(8):1270–6. This study suggests that HPA dysregulation may result in brain abnormalities through associations with metabolic abnormalities.
Acknowledgment
F. Rutters has received grant support from Marie Curie FP7 fellowship.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutters, F., La Fleur, S., Lemmens, S. et al. The Hypothalamic-Pituitary-Adrenal Axis, Obesity, and Chronic Stress Exposure: Foods and HPA Axis. Curr Obes Rep 1, 199–207 (2012). https://doi.org/10.1007/s13679-012-0024-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-012-0024-9