Abstract
Timing of exposure to lifestyle factors that influence energy balance may differentially affect colorectal cancer (CRC) risk and prognosis. Caloric restriction in youth and short stature, as markers of early-life exposures, have shown to decrease CRC risk, whereas large body size and low physical activity levels in adulthood are established risk factors for CRC. Regarding prognosis, overweight, sarcopenia, and their co-occurrence (sarcopenic obesity) may negatively influence the health and quality of life of CRC survivors. There is mechanistic support for disruption of the mammalian target of rapamycin (mTOR) pathway as an underlying mechanism possibly driving these associations, because mTOR integrates signals from growth factors, nutrients, mutagens, and hormones to induce cell proliferation, resistance to apoptosis, and autophagy. However, epidemiologic evidence connecting mTOR to energy-balance-related CRC throughout the lifespan is scarce. This perspective proposes how multidimensional molecular epidemiologic studies can shed light on the etiology and prognosis of energy-balance-related CRC.
Similar content being viewed by others
Introduction
During the course of the past century, epidemiological studies have been invaluable for advancing our knowledge about modifiable risk factors for cancer. The influence of indicators of energy imbalance, such as a high body mass index and physical inactivity, on colorectal cancer (CRC) risk has been some of the most convincing evidence to emerge from such research [1]. Interestingly, increasing support also is emerging for the importance of energy balance throughout the lifespan for both cancer risk and prognosis [2••, 3].
A challenge of contemporary epidemiology is to determine the common molecular processes through which risk factors operate [4]. There is now strong evidence to suggest that the mammalian target of rapamycin (mTOR) plays a critical role in human health and aging; a number of chronic diseases are associated with disrupted mTOR signaling, including type 2 diabetes, obesity, metabolic syndrome, and several types of cancer, including CRC [5, 6••, 7••].
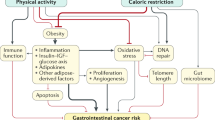
The mechanisms of mTOR signaling are well described, and animal studies have offered mechanistic insights supporting a role of mTOR in the association between energy balance and cancer development. However, epidemiologic data regarding the connection between mTOR and CRC are scarce, because few studies are large enough to consider small subgroups or do not yet have information on molecular and genetic endpoints in tumors. As more and more studies around the world are reaching the potential to gather this data, we offer a perspective of how future research could proceed, ideally by combining a number of research questions in one study. Considering the association between energy balance and CRC risk in a multidimensional fashion may not only shed light on the etiology and prognosis of CRC, but also on shared mechanisms underlying both CRC and other diseases. This can be accomplished by accounting for multiple indicators of energy exposure, including the timing of this exposure throughout the lifespan, as well as molecular and genetic characteristics of the tumor with respect to mTOR.
Lifestyle Factors Throughout the Lifespan that Influence CRC Incidence and Prognosis
Energy balance refers to the relationship between energy expenditure and energy intake. Energy imbalance resulting from more energy intake than expenditure, as reflected through overweight and obesity, is known to increase the risk of CRC [1, 8–14]. In contrast, physical activity, which can normalize this type of energy imbalance, has been shown to decrease the risk of CRC [1, 15–21]. There is no doubt that intra-abdominal (visceral) fat increases inflammatory responses and circulating estrogens, and decreases insulin sensitivity [22]. As a proxy measure for visceral adiposity, a high waist circumference has been associated with CRC risk in epidemiologic studies, independent of body mass index (BMI) [10, 23, 24]. Unique data from periods of reduced food availability, such as the Dutch Hunger Winter and World War 2 [25–32], support animal studies suggesting that caloric restriction, which can induce negative energy balance (less energy intake than expenditure), is one of the most potent interventions for preventing cancer [33–37]. Furthermore, timing of exposure to factors relating to energy balance may be important for modifying cancer risk.
Individuals who experienced caloric restriction in childhood and adolescence have a decreased risk of CRC later in life compared to those who did not experience it [27, 29, 31, 32, 38]. In contrast, body fatness during childhood [39] and adult-attained height, as a proxy measure for environmental and nutritional exposures that occurred in utero and in early childhood [40], are associated with an increased risk of CRC and/or colorectal adenomas [1, 10, 23, 39, 41].
With the aging of the population, the number of individuals living with a diagnosis of CRC (CRC survivors) is increasing. However, the role of energy balance in determining the prognosis and quality of life of survivors, an increasingly aging population, is still understudied [42]. The favorable effect of physical activity on quality of life in cancer survivors, including those with CRC [43], suggests that energy balance is relevant for healthy survivorship in this group as well. Although evidence is slowly accumulating for body fatness (usually assessed at or before diagnosis, but rarely after treatment) to have detrimental effects on survival, recurrence, and comorbidities in cancer survivors [44••, 45], the influence of lean body mass, especially loss of skeletal muscle mass (sarcopenia), on the health and quality of life of CRC survivors remains underinvestigated. Considerable variability in the body composition of CRC survivors has been noted, especially with respect to cachexia and sarcopenic obesity, i.e., muscle wasting without loss of adipose tissue [46, 47]. Sarcopenia leads to muscle function impairments, physical activity limitations, and restrictions in societal participation. Besides tumor-related hypermetabolism (increased resting energy expenditure, muscle protein catabolism) and inflammation, the balanced process of muscle protein turnover chiefly depends on nutrition and physical activity [48]. Both low-energy input (food intake, mainly low-protein nutrition) and output (physical inactivity, mainly few strength-training-type activities) contribute to progressive muscle wasting.
The mTOR Pathway in Energy-Related CRC
In a recent review, Chen identified multiple signaling pathways involved in obesity-associated cancer, and ultimately postulated that mTOR activity could be the key to unlocking the signal pathway changes that occur in the development of such cancers [49]. mTOR is a highly evolutionary conserved serine/threonine kinase that controls cell growth and metabolism in response to nutrients (e.g., amino acids), growth factors (e.g., insulin, insulin-like growth factor (IGF)-1, acting through phosphatidylinositol 3-kinase (PI3K)), cellular energy (ATP acting through inhibition of adenosine monophosphate-dependent protein kinase (AMPK)), and stress (e.g., hypoxia and DNA damage) [7••, 50]. mTOR is at the interface between growth and starvation. It positively regulates anabolic processes, such as transcription, protein synthesis, ribosome biogenesis, nutrient transport, and mitochondrial metabolism. At the same time, mTOR negatively regulates catabolic processes, such as mRNA degradation, ubiquitin-dependent proteolysis, autophagy, and apoptosis. It also has been shown that sirtuin-1 (SIRT-1), which regulates energy efficiency during caloric restriction, may negatively regulate mTOR in response to nutrients and cellular stress [51].
The PI3K-protein kinase B(Akt)-mTOR signaling pathway includes several established proto-oncogenes (e.g. PI3K, v-akt murine thymoma viral oncogene homolog 1 (AKT1)) and tumor suppressor genes (e.g. phosphatase and tensin homolog (PTEN)) [7••, 52]. The PTEN protein antagonizes PI3K function and negatively regulates Akt/mTOR activity [44••]. Epidemiological data suggest that sporadic mutation or deregulation of PI3K, Akt and PTEN are among the most prevalent alterations in human cancer [7••, 54]. Recently, Gulhati et al. reported that activation of PI3K-Akt is linked to growth and progression of CRC [55], which supports their previous finding that mTOR kinase can regulate CRC tumorgenesis [56].
A plausible mechanistic link between energy balance, mTOR, and CRC development is insulin-like growth factor 1 (IGF-1) upstream from mTOR [57]. Evidence from animal models supports the idea that reduction in circulating free IGF-1 is associated with the anticancer effects of caloric restriction [33, 58]. Epidemiological research has shown that hyperinsulemia, which decreases IGF-1 binding protein 3 (IGFBP3) and consequently increases circulating IGF-1, and diabetes both increase the risk of CRC [59–61]. Activation of mTOR has emerged as a critical event in rendering insulin receptor substrate (IRS)-1 and IRS-2 unresponsive to insulin and IGF-1 [62]. Therefore, proper regulation of the PI3K-Akt-mTOR signaling pathway is critical for the prevention of insulin resistance. Further evidence supporting this link is that excess body fat is associated with both high serum levels of IGF-1 and enhanced induction of the PI3K-Akt-mTOR signaling pathway, whereas caloric restriction is associated with reduced signaling as a result of decreased circulating levels of IGF-1 [34, 63].
There are two functionally and structurally distinct complexes which contain mTOR: mTOR complex 1 (mTORC1), which is rapamycin sensitive, and mTORC2, which is rapamycin insensitive. Although both are relevant for carcinogenesis, mTORC1 is thought to be influenced by energy balance, specifically energy restriction [2••, 7••]. Molecular mediators involved in muscle wasting likely include disruption of the mTORC1 pathway. This energy balance-related pathway is a crucial regulator of protein synthesis in response to energy input (nutrient intake) and output (physical activity). Downregulated mTORC1 signaling results in reduced protein translation and promotes protein breakdown via autophagy [7••, 64]. Decreased levels of circulating amino acids, glucose, insulin, and IGF-1 reduce mTORC1 signaling. Research on sarcopenia underscores that probably both essential amino acid availability, especially leucine, and resistance exercise counteract sarcopenia through acute regulation of mTORC1 signaling [7••, 48, 65–67]. It also is noteworthy that metformin, the initial glucose lowering therapy for type 2 diabetic patients, has been associated with a significantly lower risk of CRC in type 2 diabetic patients in a recent meta-analysis [68]. The mechanism of action of metformin is believed to be through activation of AMPK and hence inhibition of mTORC1 signaling. Tissue specificity of mTORC1 signaling has yet to be determined. It is plausible that epigenetic changes, such as methylated IGFBP genes in CRC, may disrupt mTORC1 signaling in CRC cells.
Toward a Multidimensional Molecular Epidemiologic Approach
We propose a multidimensional molecular epidemiologic approach to further unravel the role of the mTOR signaling pathway in energy-balance-related CRC etiology and prognosis throughout life (Fig. 1). Ideally, simultaneous investigation of a range of exposures and their joint effects, the timing of exposures, and diverse molecular biomarkers of the mTOR pathway is considered in future studies.
Consider a Range of Exposures and Their Joint Effects
In general, it is difficult to account accurately for energy balance in epidemiological studies, because energy balance is maintained by a complex system involving multiple pathways and is dependent on genetic factors. The variables used in epidemiological studies, such as BMI, waist circumference, caloric intake, and physical activity status, are not sufficient on their own to describe overall energy balance status [58]. Considering joint effects can help paint a more comprehensive picture than considering single variables in isolation. Both BMI and waist circumference have been reported to show interactions with physical activity levels in relation to CRC [10, 69, 70]. Such joint effects, which have not yet been investigated systematically, can reveal associations that may remain masked when focusing on just one aspect of energy balance.
In addition, investigating both ends of the exposure spectra throughout the lifespan can reinforce our knowledge of underlying pathways. As reviewed earlier, both caloric excess and caloric restriction, as well as sarcopenic obesity in CRC survivors, are associated with either cancer risk or prognosis, respectively, in part through their influence on mTOR signaling. Therefore, with respect to untangling the role of mTOR in the association between energy balance and CRC, and in terms of creating prevention strategies, it is most informative to consider joint associations between exposure variables within studies if possible or otherwise consider literature from different exposure spectra.
Consider the Timing of Exposures
Studying the timing of exposure can shed valuable insights into cancer etiology. Epidemiological studies have shown that timing of exposure, both in terms of the life phase during which exposure occurs [27, 29, 31, 32, 38, 39], and in terms of changes in exposure over time [71], may be important for modulating CRC risk. Examples from animal models of other cancer types support this idea with respect to mTOR signaling [72, 73].
Childhood obesity is now a major health concern, but the long-term consequences of being obese during this critical phase of growth and development remain largely undetermined. To our knowledge, no study has investigated the role of indicators of energy balance early in life and the effect of mTOR signaling in cancer later in life. However, evidence from a rat study investigating radiation exposure early in life supports that early life environmental exposure may have mechanistic implications for mTOR. In this study, Kokubo et al. [72] assessed the risk of renal cell carcinoma in rats irradiated at various time points in the gestational and postnatal period. At 27 weeks of age, kidneys were examined for proliferative lesions. Compared with unirradiated controls, adenoma and adenocarcinoma development were evident in perinatally irradiated kidneys. Furthermore, these malignant changes were associated with mTOR activation in tumors, suggesting that early life environmental exposure has implications for mTOR signaling.
With respect to changes in exposure over time, a recent study by De Angel et al. [73] investigated the impact of weight normalization in ovariectomized female mice on breast cancer development. Mice were fed a control diet, a calorie reduced (CR) diet, or an obesity inducing (DIO) diet. At 17 weeks, the mice on the obesity-inducing diet were switched to a control diet, resulting in obese mice decreasing to a similar weight to the controls by week 20. Relative to controls, CR mice had decreased, and DIO mice had increased serum IGF-1 and phosphorylation of Akt/mTOR pathway components. Of relevance to the point at hand is that weight normalization in obese mice did not immediately reverse tumor progression or Akt/mTOR activation. If replicated in humans, this finding could have implications for prevention strategies.
Consider Diverse Molecular Biomarkers
Biomarkers of Exposure
Assessing exposure in large-scale, prospective, epidemiologic studies is a major challenge and assessing early life-time exposures is even more challenging. Biomarkers related to altered energy balance during different phases of life could lend support to observed associations between indicators of energy balance and CRC in epidemiological studies. Multiple cancer risk factors have been identified in relation to obesity, including increased blood levels of insulin, IGF1, estrogen, proinflammatory cytokines, adipokines, and other inflammatory markers [4, 49, 60, 61, 74–76]. As reviewed by Chen [49], animal studies have shown that such factors exert their effects by activating multiple signaling pathways, including PI3K-Akt and IGF signaling pathways, which in turn lead to the activation of mTOR.
Exciting developments and opportunities should be monitored for potential application in epidemiologic studies. For example, gut microbial enterotypes have recently been associated with long-term dietary patterns, specifically protein and animal fat (Bacteroides) versus carbohydrates (Prevotella) [74]. In a study comparing the fecal microbiota of children living in Europe and rural Burkina Faso, where the diet is high in fiber content, significant differences in gut microbiota between the two groups were observed, for example, with an abundance of Prevotella bacteria in the children from Africa [75]. These studies are just emerging and need to be replicated, but the composition of gut microbiota may be an interesting future tool to fingerprint early-life and long-term exposures, including the diet, and their role in pathways, such as the mTOR pathway to CRC cancer.
Biomarkers of Outcome
One of the most exciting developments in modern epidemiology is the emergence of the field of molecular pathological epidemiology. As Ogino et al. describe, a strength of this type of research is that germ line genetic variants and molecular alterations in the tumor can be studied simultaneously, and as such, the specificity of the relationship between genetic variants and tumor molecular alterations can provide additional evidence to support a causal effect of the putative cancer susceptibility allele [76]. In addition, the molecular signatures of tumors can be viewed as fingerprints of prior internal and external exposures, because the timing of molecular events in carcinogenesis are known to differ, with epigenetic changes occurring relatively early in carcinogenesis. In the Netherlands Cohort Study on diet and cancer, early-life exposure to energy restriction was inversely associated with CRC risk according to the degree of promoter hypermethylation in a number of cancer-related genes [38]. This result suggests that exposure to severe malnutrition during youth can protect from persistent epigenetic changes that later influence CRC development.
Biomarkers of Susceptibility
Genetic variants can be viewed as time-independent biomarkers of pathway involvement [77]. Selecting genetic variants from pathways related to mTOR signaling and investigating their interaction with determinants of energy balance in relation to CRC is thereby another promising avenue for molecular epidemiologic research in this area.
In 2009, Slattery et al. proposed the “convergence of hormones, inflammation, and energy-related factors” (CHIEF) pathway, to provide a mechanistic explanation for the convergent effects of inflammation, hormones, and energy-related factors, such as obesity, physical activity, and energy intake on colorectal carcinogenesis [4]. Supported by molecular and epidemiological evidence, there are two major arms of the pathway, one of which contains mTOR. Using data from the Northern California Kaiser Permanente Medical Care Program (KPMCP) and the Twin Cities Metropolitan area of Minnesota, Slattery et al. investigated genetic variation in the CHIEF pathway and showed that variation in mTOR altered the risk of CRC, even after adjustment for multiple comparisons [78]. Intriguingly, when molecular tumor markers were taken into account, the strongest associations for mTOR were observed for tumors characterized by microsatellite instability (MSI). This suggests that through the CHIEF pathway, mTOR may influence risk through epigenetic changes, and shows the importance of considering both the genetic and molecular characteristics of the tumor.
The benefit of studying gene-environment interactions can be seen in a recent case-control study by Lin et al. [70], in which the association between energy balance and risk of bladder cancer was investigated in 803 cases and matched controls by assessing the joint effects of genetic variants in PI3K-Akt-mTOR pathway genes with indicators of energy balance. Although several, large, population-based studies have reported genetic variability of the mTOR pathway with respect to cancer risk [52, 78, 79], to our knowledge, the study of Lin et al. is the only study to date to consider energy balance additionally in this equation. To explore how unfavorable genotypes in the mTOR pathway may modify the association between energy balance and cancer risk, the authors created a sum of unfavorable genotypes to conduct a stratified analysis (0–4 unfavorable genotypes, 5–6 unfavorable genotypes, and ≥7 unfavorable genotypes). Those subjects with the worst “energy balance” category (high caloric intake, low physical activity, high number of unfavorable genotypes) had a significantly higher risk of bladder cancer compared with those individuals with the most favorable energy balance category (low caloric intake, intensive physical activity, low number of unfavorable genotypes). Intriguingly, this effect was stronger than when caloric intake and physical activity were considered independently from each other.
Conclusions
Although with small sample sizes it may be a challenge for individual cohorts to combine our suggested considerations into one study, there is emerging potential to pool data from multiple studies utilizing a molecular pathological epidemiology approach to research [80, 81]. Furthermore, besides increased statistical power when data from multiple studies are appropriately combined, the findings from such pooled cohorts will have increased generalizability and external validity. Such collaboration and cooperation will serve to advance population science and public health [81]. It also is intriguing to speculate about how future studies could simultaneously focus on multiple diseases that are interlinked through the mTOR signaling pathway and shared risk factors. For example, energy imbalance is a risk factor for several types of cancer in addition to CRC, which also have been shown to demonstrate impaired mTOR signaling. These include breast cancer [34, 73, 82], bladder cancer [70, 79], and renal cell carcinoma [53]. One may hypothesize that despite the different tumor types, a similar underlying mechanism is at play. Therefore, to find out how molecular and genetic aspects of mTOR signaling pathway genes modify the association between energy balance and cancer, it may be informative and statistically more powerful to consider cancer as a single endpoint comprised of a number of specific cancers that may share etiology, such as obesity-related cancers. Furthermore, as noted earlier, dysregulated mTOR signaling is associated with several diseases that are associated with CRC, such as metabolic syndrome and type 2 diabetes [5, 6••, 7••]. A truly multidimensional approach, and one that would shed valuable insight on etiology, would be to simultaneously consider these related diseases so that the pathway to disease is being investigated rather than individual disease endpoints.
In conclusion, defects in mTOR signaling may be the key to unlocking the reported associations between overweight, obesity, caloric restriction throughout life, and CRC. We have presented a perspective of how future epidemiological studies could proceed to glean the most mechanistic information about such biological pathways. Considering genetic and molecular aspects of the mTOR signaling pathway in conjunction with indicators of energy balance at different periods of life will result in a better understanding of CRC etiology and prognosis, which is necessary for fine tuning prevention strategies.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AIRC; 2007.
•• Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res. 2012;5:1260–72. This article provides a description of mechanisms that underlie the obesity-cancer relationships and discusses opportunities for primary to tertiary prevention.
Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30:3697–704.
Slattery ML, Fitzpatrick FA. Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev Res. 2009;2:922–30.
Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–55.
•• Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. This is another review on the mechanisms linking mTOR signaling to various diseases and discussing pharmacological approaches.
•• Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. This article features a comprehensive review of mTOR regulators, the action of mTOR and its role in cancer, diabetes and ageing.
Campbell PT, Jacobs ET, Ulrich CM, et al. Case-control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102:391–400.
Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–206.
Hughes LA, Simons CC, van den Brandt PA, et al. Body size and colorectal cancer risk after 16.3 years of follow-up: an analysis from the Netherlands Cohort Study. Am J Epidemiol. 2011;174:1127–39.
Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30.
Oxentenko AS, Bardia A, Vierkant RA, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer Prev Res. 2010;3:1608–20.
Wang Y, Jacobs EJ, Patel AV, et al. A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence. Cancer Causes Control. 2008;19:783–92.
Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65.
Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009;11:547–63.
Moradi T, Gridley G, Bjork J, et al. Occupational physical activity and risk for cancer of the colon and rectum in Sweden among men and women by anatomic subsite. Eur J Cancer Prev. 2008;17:201–8.
Nilsen TI, Romundstad PR, Petersen H, et al. Recreational physical activity and cancer risk in subsites of the colon (the Nord-Trondelag Health Study). Cancer Epidemiol Biomark Prev. 2008;17:183–8.
Parent ME, Rousseau MC, El-Zein M, et al. Occupational and recreational physical activity during adult life and the risk of cancer among men. Cancer Epidemiol. 2011;35:151–9.
Spence RR, Heesch KC, Brown WJ. A systematic review of the association between physical activity and colorectal cancer risk. Scand J Med Sci Sports. 2009;19:764–81.
Wolin KY, Lee IM, Colditz GA, et al. Leisure-time physical activity patterns and risk of colon cancer in women. Int J Cancer. 2007;121:2776–81.
Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–6.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91.
Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31.
Pischon T, Nöthlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–45.
Dirx MJ, van den Brandt PA, Goldbohm RA, et al. Diet in adolescence and the risk of breast cancer: results of the Netherlands Cohort Study. Cancer Causes Control. 1999;10:189–99.
Dirx MJ, van den Brandt PA, Goldbohm RA, et al. Energy restriction in childhood and adolescence and risk of prostate cancer: results from the Netherlands Cohort Study. Am J Epidemiol. 2001;154:530–7.
Dirx MJ, van den Brandt PA, Goldbohm RA, et al. Energy restriction early in life and colon carcinoma risk: results of The Netherlands Cohort Study after 7.3 years of follow-up. Cancer. 2003;97:46–55.
Elias SG, Peeters PH, Grobbee DE, et al. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96:539–46.
Hughes LA, van den Brandt PA, Goldbohm RA, et al. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: results from the Netherlands Cohort Study. Int J Epidemiol. 2010;39:1333–44.
Schouten LJ, van Dijk BA, Lumey LH, et al. Energy restriction during childhood and early adulthood and ovarian cancer risk. PLoS One. 2011;6:e27960.
Svensson E, Grotmol T, Hoff G, et al. Trends in colorectal cancer incidence in Norway by gender and anatomic site: an age-period-cohort analysis. Eur J Cancer Prev. 2002;11:489–95.
Svensson E, Moller B, Tretli S, et al. Early life events and later risk of colorectal cancer: age-period-cohort modelling in the Nordic countries and Estonia. Cancer Causes Control. 2005;16:215–23.
Hursting SD, Lavigne JA, Berrigan D, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52.
Moore T, Beltran L, Carbajal S, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res. 2008;1:65–76.
Kritchevsky D. Colorectal cancer: the role of dietary fat and caloric restriction. Mutat Res. 1993;290:63–70.
Kritchevsky D, Klurfeld DM. Influence of caloric intake on experimental carcinogenesis: a review. Adv Exp Med Biol. 1986;206:55–68.
Weindruch R, Albanes D, Kritchevsky D. The role of calories and caloric restriction in carcinogenesis. Hematol Oncol Clin N Am. 1991;5:79–89.
Hughes LA, van den Brandt PA, de Bruine AP, et al. Early life exposure to famine and colorectal cancer risk: a role for epigenetic mechanisms. PLoS One. 2009;4:e7951.
Nimptsch K, Giovannucci E, Willett WC, et al. Body fatness during childhood and adolescence, adult height, and risk of colorectal adenoma in women. Cancer Prev Res. 2011;4:1710–8.
Okasha M, Gunnell D, Holly J, et al. Childhood growth and adult cancer. Best Pract Res Clin Endocrinol Metab. 2002;16:225–41.
Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–94.
Jacobsen PB, Jim HS. Consideration of quality of life in cancer survivorship research. Cancer Epidemiol Biomark Prev. 2011;20:2035–41.
Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105 suppl 1:S52–73.
•• Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomark Prev. 2012;21:1244–59. This article summarizes the discussion of a workshop and covers mechanisms, complexities of studying and interpreting observational and intervention research and discusses future directions.
Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2012;92:471–90.
Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700.
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601.
Walker DK, Dickinson JM, Timmerman KL, et al. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc. 2011;43:2249–58.
Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–70.
Hall MN. mTOR-what does it do? Transplant Proc. 2008;40:S5–8.
Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199.
Campa D, Husing A, Stein A, et al. Genetic variability of the mTOR pathway and prostate cancer risk in the European Prospective Investigation on Cancer (EPIC). PLoS One. 2011;6:e16914.
Azim H, Azim Jr HA, Escudier B. Targeting mTOR in cancer: renal cell is just a beginning. Target Oncol. 2010;5:269–80.
Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510.
Gulhati P, Bowen KA, Liu J, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56.
Gulhati P, Cai Q, Li J, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009;15:7207–16.
Moore T, Checkley LA, DiGiovanni J. Dietary energy balance modulation of epithelial carcinogenesis: a role for IGF-1 receptor signaling and crosstalk. Ann N Y Acad Sci. 2011;1229:7–17.
Hursting SD, Lashinger LM, Wheatley KW, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–69.
Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–20S.
Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106.
Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87.
Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403.
Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–9.
Weigl LG. Lost in translation: regulation of skeletal muscle protein synthesis. Curr Opin Pharmacol. 2012;12:377–82.
Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2010;3:90–101.
Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signaling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6.
Dickinson JM, Fry CS, Drummond MJ, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–62.
Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323–8.
Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer-beyond physical activity. Cancer Res. 1997;57:75–80.
Lin J, Wang J, Greisinger AJ, et al. Energy balance, the PI3K-AKT-mTOR pathway genes, and the risk of bladder cancer. Cancer Prev Res (Phila). 2010;3:505–17.
Morois S, Mesrine S, Josset M, et al. Anthropometric factors in adulthood and risk of colorectal adenomas: the French E3N-EPIC prospective cohort. Am J Epidemiol. 2010.
Kokubo T, Kakinuma S, Kobayashi T, et al. Age dependence of radiation-induced renal cell carcinomas in an Eker rat model. Cancer Sci. 2010;101:616–23.
De Angel RE, Conti CJ, Wheatley KE, et al. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2012.
Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8.
De Fellipo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107:14691–6.
Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2010;60:397–411.
Le Marchand L, Wilkens LR. Design considerations for genomic association studies: importance of gene-environment interactions. Cancer Epidemiol Biomark Prev. 2008;17:263–7.
Slattery ML, Herrick JS, Lundgreen A, et al. Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis. 2010;31:1604–11.
Chen M, Cassidy A, Gu J, et al. Genetic variations in PI3K-AKT-mTOR pathway and bladder cancer risk. Carcinogenesis. 2009;30:2047–52.
Hughes LA, Williamson EJ, van Engeland M, et al. Body size and risk for colorectal cancers showing BRAF mutations or microsatellite instability: a pooled analysis. Int J Epidemiol. 2012; doi: 10.1093/ije/dys055.
Ogino S, Giovannucci E. Commentary: lifestyle factors and colorectal cancer microsatellite instability–molecular pathological epidemiology science, based on unique tumor principle. Int J Epidemiol. 2012. doi:10.1093/ije/dys076.
Nogueira LM, Dunlap SM, Ford NA, et al. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. 2012;19:57–68.
Acknowledgments
M.J.L. Bours is supported by a grant from the Alpe d’HuZes Foundation from the Dutch Cancer Fund (Grant Number UM 2010-4867). C.C.J.M. Simons is supported by a grant from the Dutch Cancer Fund (Grant Number UM 2009-4281).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Weijenberg, M.P., Hughes, L.A.E., Bours, M.J.L. et al. The mTOR Pathway and the Role of Energy Balance Throughout Life in Colorectal Cancer Etiology and Prognosis: Unravelling Mechanisms Through a Multidimensional Molecular Epidemiologic Approach. Curr Nutr Rep 2, 19–26 (2013). https://doi.org/10.1007/s13668-012-0038-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-012-0038-7
Keywords
- mTOR pathway
- Colon
- Rectum
- Neoplasm
- Cancer risk
- Etiology
- Cancer prognosis
- Cancer survivor
- Diet
- Physical activity
- Energy balance
- Sarcopenia
- Overweight
- Obesity
- Dietary protein
- Energy restriction
- Adolescent
- Childhood
- Elderly
- Molecular epidemiology
- Epigenetic
- Genetic variation
- Somatic mutations
- Immunohistochemical expression
- Biomarkers
- Gut microbiota