Abstract
Purpose of review
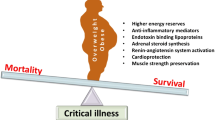
Obesity has been associated with increased incidence of diabetes, atherosclerotic disease, numerous cancers, and other comorbidities. Given the increased comorbidities and challenges associated with bedside care of the critically ill obese patient, the expectation would be worse overall clinical outcomes. However, it has been noted that there are improved outcomes in critically ill patients who are overweight or mildly obese compared to their underweight and morbidly obese counterparts. This has been termed the obesity paradox. The purpose of this article is to discuss the validity of the obesity paradox and to explore potential explanations for this seemingly illogical association.
Recent findings
Body mass index (BMI) represents a crude measurement of an individual’s metabolic health and may be, in part, responsible for the observed obesity paradox. Alternative markers, including lean muscle mass:adipose ratios, could better clarify which patients are prone to poor outcomes. In the event that the obesity paradox is not attributable to statistical aberrancies associated with the utilization of BMI, emerging findings regarding the role of the microbiome and systemic effects of adipokines during critical illness represent potential explanations for improved outcomes in this patient population.
Summary
The explanations for the observed obesity paradox are likely multifactorial. Obesity, as defined by BMI, may correlate poorly with overall metabolic health, and there may be better markers for assessment. Alternatively, the gastrointestinal microbiome and variable systemic effects of adipokines may truly contribute to improved overall survival in critically ill obese patients.
Similar content being viewed by others
Introduction
Obesity currently affects greater than 30% of the population in the USA and has been associated with an increased incidence of diabetes, atherosclerotic disease, and malignancy [1]. In 2008, obesity-related healthcare costs were estimated at $147 billion and this likely represents an underestimation of the true figure [2]. Comorbidities associated with obesity are common, result in significant morbidity and mortality, and are expensive to manage. The parameter most commonly used to define obesity is body mass index (BMI), a number calculated by dividing a person’s mass in kilograms by the square of their height in meters. The value, expressed in kg/m2, can then be categorized into underweight (less than 18.5), normal weight (18.5–24.9), overweight (25–29.9), class I (30–34.9), class II (35–39.9), class III (>40), and class IV (>50) obesity. The BMI provides a quantitative and easily obtainable variable to follow in the clinical and research settings [3].
As in the general population, one third of patients in the intensive care unit (ICU) are obese [4]. Obesity presents multiple challenges in the provision of care in the ICU including the management of associated comorbidities, procedural difficulties, and proclivities for airway management issues and ventilator weaning difficulties. Given these issues that have been acknowledged in detail in prior publications, obese patients would be expected to have worse clinical outcomes in critical illness [5, 6]. However, although results have been mixed, multiple studies have demonstrated a survival benefit in the overweight and class I obese patients when compared to non-obese populations [7,7,8,9,11]. This observation has been termed the “obesity paradox.” The reason for the obesity paradox is unclear. Several potential explanations for the ICU obesity paradox exist: (1) The physiology associated with obesity provides some beneficial inflammatory “preconditioning” that prepares patients for inflammation associated with critical illness or (2) increased energy reserves are eventually mobilized and utilized in a beneficial way in the setting of critical illness, or (3) the paradox is an aberrancy stemming from the incomplete picture with regard to metabolic health resulting from using BMI alone.
Obesity in the ICU
As previously mentioned, approximately one third of ICU patients are obese [4]. Obesity creates challenges in the care of the critically ill patient. Landmarks can be more difficult to assess for bedside procedures including intravenous access and chest tubes. Endotracheal intubation and airway management can be more difficult due to the excess of soft tissue obstructing the field of view [12]. Obesity can make ventilator weaning more difficult and excess weight and skin can increase risk for skin breakdown and ulceration [13]. Obesity is a known cardiac risk factor and is associated with greater risk for hypertension, hyperlipidemia, atherosclerotic disease, and myocardial infarction [14, 15]. These are a few examples that would lead one to assume that obesity would contribute to worse outcomes in critical illness [16, 17]. Despite the above concerns, numerous studies have shown a decrease in overall mortality in overweight and obese patients. In a meta-analysis by Flegal et al., overweight and obese patients had a 6 and 5% decrease in all-cause mortality, respectively [18]. The obesity paradox has been demonstrated in non-dialysis-dependent kidney disease, cirrhosis, and community-acquired pneumonia [19,19,21]. As previously mentioned, these studies used BMI to define obesity.
The paradox as a statistical aberrancy
Several issues contribute to the potential that the perceived paradox is a statistical aberrancy due to the nature of utilizing BMI as the defining characteristic of obesity. In addition, the obesity paradox does not seem to apply to all populations, suggesting that extrapolating conclusions from mixed populations may be fraught with error.
Defining obesity
Although BMI remains the predominant variable utilized in clinical practice, other metrics exist for defining obesity such as waist to hip ratio, waist circumference, sagittal abdominal diameter, and body fat percentage measurements [22, 23]. BMI measures body weight, but provides little information regarding the constituents (fat, muscle, etc.) comprising the weight. Antonopoulos and colleagues discuss that BMI does not take into account sarcopenic obesity, lean body mass, or cardiorespiratory fitness. They suggest that the obesity paradox may be better termed a ‘BMI paradox’ and recommend using more specific means of categorizing patients when analyzing outcomes [24]. Obesity lacks uniformity and encompasses a variety of body types not well elucidated by BMI alone. The metabolically healthy obese (MHO) population is characterized by obesity without metabolic derangements, i.e., dyslipidemia, metabolic syndrome, or insulin resistance. Obesity with decreased lean muscle mass (sarcopenia), termed sarcobesity by Parr et al., represents another body type associated with obesity [25]. Sarcobesity may already be present in the morbidly obese due to a higher adipose/lean muscle mass ratio and it is more pronounced in critical illness because of lean muscle (fat free) mass wasting. This may posit one simplified explanation for what is a much more complex issue. The ICU survival benefit may favor those with higher lean body mass (or fat free mass), hence favoring a subset of the overweight and obese. Those who are underweight and morbidly obese often have sarcopenia and reduced overall fat free mass to fat mass ratios, placing them at higher risk in critical illness. DeSchutter et al. took 47,866 patients in a retrospective analysis calculating patients lean mass index, body fat, and BMI and were able to show that lean mass is protective, but survival benefit is lost in the setting of sarcopenic obesity [26]. In addition, a prospective observational study (n = 403) evaluated sagittal abdominal diameter at ICU admission with the primary outcome measure being mortality. After adjustment for a number of variables (age, APACHEII score, etc.), multivariate analysis demonstrated an increased risk of mortality in those with elevated abdominal obesity (OR 2.12 95% CI, 1.25–3.60), whereas obesity alone (as defined by BMI >30 kg/m2) was not an independent risk factor for death [27]. Alternatively, Ortega et al. suggest that there is consistent evidence supporting the obesity paradox in cardiovascular disease and that BMI is a stronger predictor of cardiovascular mortality than quantitative measures of fat mass. They discuss the MHO phenotype as a potential explanation for the obesity paradox and believe this classification should be used to further clarify and augment research [28].
The heterogeneity of the ICU
An additional limitation of the observed obesity paradox in the ICU population is the heterogeneous nature of ICU populations. The three meta-analyses demonstrating the obesity paradox in the ICU are mixed populations (medical, trauma, and surgical) [9,9,11]. Each of these groups has significant individual heterogeneity (for example, traumatic brain injury versus penetrating abdominal trauma in the trauma population), and when combined involve multiple layers of heterogeneity that confound any generalized observations. A recent meta-analysis evaluated trauma patients independent of other ICU types (surgical and medical) [29]. The primary aim of the analysis was to compare non-obese (<30 kg/m2) patients with obese patients (>30 kg/m2) patients admitted to trauma units. The analysis included 18 studies with a total of 7751 obese patients (17% of the pooled population). Obese patients had increased risk of complication and increased ICU length of stay compared to non-obese patients with no differences in mechanical ventilation, injury severity score (ISS), or hospital length of stay. The overall mortality of obese patients was 7.7 versus 4.7% for the non-obese patients with an overall effect OR 1.45. Obesity was a risk factor in mortality due to blunt trauma (OR 2.02; 95% CI 1.69–2.40; p < 0.001; I2 = 88.1%) in studies that included this data. In addition, there was no mortality difference between obese and non-obese patients with an ISS ≥15 [29]. Hypovolemic and hemorrhagic shock are markedly different with regard to pathophysiology when compared to distributive shock (representing the majority of the medical ICU). Traumatic shock may lead to persistent vasoconstriction of small bowel vasculature with resultant multiple organ dysfunction. In an animal study, obese rats that were shocked as a result of hemorrhage had an almost complete loss of hepatic perfusion. Following resuscitation, an exaggerated inflammatory response was demonstrated in the obese animals when compared to the non-obese [30]. Regardless of the mechanism, it is clear that to include the pathophysiology of trauma ICU patients with medical ICU patients is likely not appropriate and the paradox may apply to only a subset of critically ill patients.
Physiologic explanations for the paradox
Given the assumption that the obesity paradox is not a statistical aberrancy, there are potential explanations for the improved outcomes observed in obese patients. Among these explanations are pro-inflammatory priming and the presence of a microbiome more suitable to withstand critical illness.
Pro-inflammatory preconditioning
Adipose tissue can be categorized as subcutaneous, peripheral, central, visceral, or intraparenchymal in nature. Central adiposity is more likely to be associated with intraparenchymal deposition of adipose tissue. Due to continuous and more direct interaction of visceral adiposity with the systemic circulation when compared to peripheral adiposity, the associated metabolic derangements and pro-inflammatory state may be more pronounced in this subset of the population. Adipose tissue is not an inert form of energy storage and is instead a metabolically active participant. There are a host of adipokines that play various roles including regulation of inflammation, attenuation of the immune response, angiogenesis, and cardioprotection. Obesity has traditionally been considered a pro-inflammatory state due to the downstream impact of increased circulating adipokines. This downstream upregulation of pro-inflammatory mediators is thought to contribute to auto-immunity (e.g., psoriasis), insulin resistance, and the metabolic syndrome [31, 32]. Although adipokines have numerous pro-inflammatory properties, some are cardioprotective and may be beneficial in the setting of critical illness. [33•, 34••]. For example, TNF alpha has two active receptors, TNFR1 and TNFR2. TNFR2 is cardioprotective triggering enhanced angiogenesis and has beneficial impact on the endothelium, hematopoetic cells, and cardiomyocytes. TNFR1, on the other hand, is present on all cells and may prohibit/limit cardiac remodeling and is generally considered pro-inflammatory. Increasing BMI may have an association with the overall balance of beneficial and detrimental adipokines that favorably prime the inflammatory response and immune system to respond in times of critical illness while also exerting a cardioprotective effect that mitigates the cardiac response to the stress of critical illness. This concept is not dissimilar from ischemic preconditioning, wherein patients with low-flow or intermittent ischemia of an affected extremity are more tolerant of complete ischemic episodes than patients with normal pre-existing perfusion. Interestingly, the concept of pro-inflammatory preconditioning has some evidence in the setting of rheumatoid arthritis (RA) where increasing BMI is associated with decreased radiographic progression of disease. Mangnus et al. examined 195 RA patients, 159 patients with other inflammatory arthritides, and 193 asymptomatic volunteers with MRI scan of metacarpophalangeal, wrist, and metatarsophalangeal joints. Increasing BMI was associated with higher MRI inflammation scores in both the asymptomatic and alternative inflammatory arthritides, but RA patients with higher BMI had decreased inflammatory scores [21]. Similarly, obese patients may have some element of pro-inflammatory priming that better prepares their metabolic machinery to respond in a protective manner in the setting of critical illness.
The microbiome of obesity
The “normal flora” of the gastrointestinal tract is an oxymoronic euphemism, as the microbiome of the gut is numerous and varies based on exogenous influences. The bacteria in the gastrointestinal tract outnumber the cells in the human body by a factor of 10 to 1. The bacteria in the gut are made up of nine phyla, but the majority fall into two phylum, Bacteroides and Firmicutes. While the causality is unclear, there is an associated change in the composition of the microbiome associated with obesity. In the setting of obesity, increased Firmicute:Bacteroides ratios have been demonstrated [35, 36]. This alteration in the microbiome seems to be perpetuated by a high fat diet. The microbiome also varies between patients with insulin resistance and type 2 diabetes mellitus and patients who are lean. The microbiome has a strong influence on obesity, diabetes, and the metabolic syndrome [37]. In addition, the microbiome plays a key role in immunomodulation. Short-chain fatty acids (SCFAs) and their metabolic byproducts have anti-inflammatory properties. SCFAs and their byproducts promote Treg development, affect cytokine production, and alter dendritic cells. Bacteria in the gut are able to maintain homeostasis by interacting with Toll-like receptors, upregulating Tregs which allow a hospitable environment for the bacteria. This interaction also increases expression of anti-inflammatory mediators such as CD 73 and CD 39 [38]. The microbiota interact with the immune system to maintain gut homeostasis in ways that have not been fully elucidated and may also play a pro-inflammatory role via interaction with Toll-like receptors, Tregs, and cytokine activity. As comprehension regarding the role of the microbiome in critical illness improves, alterations of the microbiome in the obese patient may prove to contribute in part to associated outcomes.
The impact of the obesity paradox on nutrition support strategies in the ICU
How do the above characteristics of the obese patient alter our nutrition support strategies in the ICU? The short answer is that, at current, they do not [39]. The objectives inherent in the provision of nutrition support in the ICU include maintenance of a healthy microbiome, stimulation of the immune response, and the prevention of lean body mass loss. Current recommendations for nutrition support in critically ill patients encourages the early initiation of enteral nutrition, regardless of BMI or other weight-related parameters. When estimating caloric needs, the clinician generally selects from numerous predictive equations, weight-based calculations, or indirect calorimetry. Indirect calorimetry is particularly useful in the inpatient setting in the obese population. Many predictive equations are not validated in critical illness, severe obesity, or either. Weight-based equations are useful for their simplicity, but clinicians must consider if actual body weight (ABW) or ideal body weight (IBW) is the appropriate multiplier. The American Society for Parenteral and Enteral Nutrition (ASPEN) and Society of Critical Care Medicine (SCCM) guidelines currently recommend weight-based nutrition dosing 11–14 kcal/kg ABW for BMI 30–50 but for BMI greater than 50 using IBW and targeting 22–25 kcal/kg [40••]. Overfeeding has been demonstrated to worsen outcomes, and recent trends have been to error toward underfeeding during the initial phase of critical illness [41]. Arabi et al. demonstrated that underfeeding (40–60% of goal calories) was not associated with worse overall mortality or clinical outcomes when compared to full feeding (70–100%) strategies [42•]. The mean BMI in this 894 patient study was 29 and 29.7 in the underfeeding and full feeding groups, respectively, falling within the overweight classification and just on the cusp of class I obesity. Protein provision was similar in both groups [42•]. Choi et al. demonstrated similar results in a meta-analysis evaluating effect of caloric intake on overall mortality. They found the top one third and the bottom one third of caloric intake groups had similar mortality rates, but the middle third, getting greater than 33.3% but less than 66.6% of standard caloric intake, had significantly lower overall mortality [43]. Hypocaloric feeding in the critically ill obese patient has long been the norm and it appears that feeding strategies in non-obese critically ill patients are trending toward similar strategies. Of note, “underfeeding” principles apply to caloric provision alone and refers predominately to exogenously provided carbohydrate. Protein provision, per current recommendations, should still be targeted at 2 g/kg/day IBW with BMI 30–40 and 2.5 g/kg/day IBW when BMI >40 [40••].
Conclusion
Obesity is increasingly more prevalent, affecting one third of the general population and the critically ill. Although not consistent across all ICU populations, there does appear to be a propensity for survival in the obese patient in the medical ICU despite obvious clinical challenges presented by this patient population. On the other hand, obese patients suffering from critical illness as a result of trauma do not seem to benefit from similar improved outcomes and appear to be one exception to the obesity paradox.
The endocrine properties of adipose tissue have been recognized as playing a significant role in the maintenance of homeostasis. Adipokines have pro-inflammatory potential but may also serve as cardioprotectants and have beneficial properties. These unique properties may play a more pronounced role in the setting of heart failure as opposed to other etiologies of shock and critical illness, accounting for the varying associations with obesity and clinical outcomes in different ICU populations. Although poorly understood, favorable alterations in the microbiome of the obese patient population could also be a potential contributor to improved clinical outcomes.
Alternatively, the obesity paradox may be due in part to the vagaries associated with utilizing BMI as the lone criteria by which obesity is clinically defined. Further stratification and categorization within this population has resulted in distinct sub-groups such as sarcopenic obesity and the metabolically healthy obese. Lean body mass, or fat-free mass, may be associated with improved outcomes, whereas disproportionate increases in adipose (fat mass) alone may be detrimental and not fully accounted for by BMI. Further characterization of these groups may allow for improved clarity with regard to the specific characteristics of the obese patient that contribute to outcomes following critical illness.
Regardless of the validity of the obesity paradox as a concept, nutrition support strategies in the obese patient are not markedly different from those utilized in the non-obese population. The exception to this involves the determination of resting energy expenditure and caloric goals as predictive equations are notoriously inaccurate in this population. Indirect calorimetry should be utilized when available and early initiation of enteral nutrition should be the goal. Interestingly, although the concept of high protein, hypocaloric nutrition support has long been touted in the obese population, nutrition support strategies in all critically ill patients are now trending toward similar approaches.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14.
Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28:822–31.
Pi-Sunyer FX, et al. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institute of Health Publication. 1998
Finkielman JD, Gajic O, Afessa B. Underweight is independently associated with mortality in post-operative and non-operativepatients admitted to the intensive care unit: a retrospective study. BMC Emerg Med. 2004;4(1):3.
Braun N, Hoess C, Kutz A, Christ-Crain M, Thomann R, Henzen C, et al. Obesity paradox in patients with community-acquired pneumonia: Is inflammation the missing link? Nutrition. 2016. doi:10.1016/j.nut.2016.07.016.
Pompillo CE, Pelosi P, Castro MG. The bariatric patient in the intensive care unit: pitfalls and management. Curr Atheroscler Rep. 2016;18:55.
Prescott HC, Chang VW, O’Brien Jr JM, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42(8):1766–74.
Martino JL, Stapleton RD, Wang M, et al. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140(5):1198–206.
Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–8.
Hogue Jr CW, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–70. doi:10.1007/s00134-009-1424-5.
Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity. 2008;16(3):515–21. doi:10.1038/oby.2007.102.
Cook TM, Woodall N, Frerk C. Fourth national audit project. Major complications of airway management in the UK: results of the fourth national audit project of the Royal College of Anaesthetists and the difficult airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–31.
Pelosi P, Quintel M, Malbrain ML. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg. 2007;62 Suppl 1:78–88.
Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–9.
Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60.
Leonard KL, Davies SW, Waibel BH. Perioperativemanagement of obese patients. Surg Clin North Am. 2015;95(2):379–90.
Shashaty MG, Stapleton RD. Physiological and management implications of obesity in critical illness. Ann Am Thorac Soc. 2014;11(8):1286–97.
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and metaanalysis. JAMA. 2013;309:71–82.
Ahmadi SF, Zahmatkesh G, Ahmadi E, et al. Association of body mass index with clinical outcomes in non-dialysis-dependent chronic kidney disease: a systematic review and meta-analysis. Cardiorenal Med. 2016;6:37–49.
Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. 2016;36(10):1450–6. doi:10.1111/liv.13137.
Mangnus L, Nieuwenhuis WP, van Steenbergen HW, Huizinga TW, Reijnierse M, van der Helm-van Mil AH. Body mass index and extent of MRI-detected inflammation: opposite effects in rheumatoid arthritis versus other arthritides and asymptomatic persons. Arthritis Res Ther. 2016;18(1):245.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–86. doi:10.1111/j.1467-789X.2011.00952.x. Review.
Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Investig. 2013;36(7):537–43. doi:10.3275/8943. Review.
Antonopoulos AS, Oikonomou EK, et al. From the BMI Paradox to the Obesity Paradox: the obesity-mortality association in coronary artery disease. Obes Rev.
Parr EB, Coffey VG, Hawley JA. ‘Sarcobesity’: a metabolic conundrum. Maturitas. 2013;74:109–13.
De Schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc. 2014;89:1072–9.
Paolini JB, Mancini J, Genestal M, Gonzalez H, McKay RE, Samii K, et al. Predictive value of abdominal obesity vs. body mass index for determining risk of intensive care unit mortality. Crit Care Med. 2010;38(5):1308–14. doi:10.1097/CCM.0b013e3181d8cd8b.
Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–70.
Liu T, Chen JJ, Bai XJ, Zheng GS, Gao W. The effect of obesity on outcomes in trauma patients: a meta-analysis. Injury. 2013;44(9):1145–52. doi:10.1016/j.injury.2012.10.038.
Matheson PJ, Franklin GA, Hurt RT, Downard CD, Smith JW, Garrison RN. Direct peritoneal resuscitation improves obesity-induced hepatic dysfunction after trauma. J Am Coll Surg. 2012;214(4):517–28. doi:10.1016/j.jamcollsurg.2011.12.016. discussion 528–30.
Wolk K, Sabat R. Adipokines in Psoriasis: An important link between skin inflammation and metabolic alterations. Rev Endocr Metab Disord. 2016.
Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56.
• Cave MC, Hurt RT, Frazier TH, et al. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr Clin Pract. 2008;23:16–34. Interesting paper discussing adipokines and potential role of specialized nutrition support strategies.
•• Sawicka M, Janowska J, Chudek J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol. 2016;222:581–9. Provides an excellent review of adipokines including systemic effects with particular focus on the cardiovascular system.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology—human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from nondiabetic adults. PLoS One. 2010;5:e9085.
Lobo LA, Benjamin CF, Oliveira AC. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin Transl Immunol. 2016;5:e90.
Patel JJ, Rosenthal MD, Miller KR, Codner P, Kiraly L, Martindale RG. The critical care obesity paradox and implications for nutrition support. Curr Gastroenterol Rep. 2016;18(9):45. doi:10.1007/s11894-016-0519-8.
•• McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical CareMedicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. These are evidence-based ASPEN and SCCM guidelines discussing nutrition support strategies in adult critical care, including management of nutrition support for critically-ill obese.
Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17.
• Arabi YM, Abdulaziz SA, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–408. This study demonstrated the safety of permissive underfeeding in the ICU.
Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2015;39(3):291–300.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Keith R. Miller has received compensation from Nestlé for serving as faculty in its fellowship program. Nathan Ludwig and Ryan Hurt declare no conflicts of interest with respect to authorship or publication of this manuscript.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nutrition and Clinical Care
Rights and permissions
About this article
Cite this article
Ludwig, N., Hurt, R.T. & Miller, K.R. The obesity paradox: validity and clinical implications. Curr Pulmonol Rep 6, 58–63 (2017). https://doi.org/10.1007/s13665-017-0167-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-017-0167-y