Abstract
• Key message
Xylem and soil CO 2 fluxes in coppiced oak forests increase with clonal size, suggesting larger expenditures of energy for root respiration. An imbalance between root demand and shoot production of carbohydrates may contribute to the degradation of abandoned coppices.
• Context
Our understanding of root respiration is limited, particularly in root-resprouting species with many stems and a large system of interconnected roots resulting from long-term coppicing.
• Aims
We tested the hypothesis that clone size influences the internal flux of CO2 dissolved in xylem sap (F T) from roots into the stem and soil CO2 efflux (F S) as indicators of root respiration. We predicted that large clones would exhibit higher F T per stem and F S than small clones due to larger root system per stem in large clones.
• Methods
Genetic analyses were performed to elucidate clonal grouping. F T was measured continuously for 100 days in 16 similar-sized stems of Quercus pyrenaica belonging to two large and two small clones. F S was measured in 20 clones of varying size.
• Results
F T per stem and F S were higher in large clones. F T was 2 % of the root-respired CO2 that diffused through soil to the atmosphere.
• Conclusions
Relative to other studies, the contribution of F T to root respiration was very low, pointing to large differences depending on species or site. Higher stem F T and F S in large clones compared with small clones suggest greater carbon consumption by roots in large clones, pointing to a root/shoot biomass and physiological imbalance resulting from long-term coppicing that would partially explain the degradation of currently abandoned stands of Q. pyrenaica.
Similar content being viewed by others
1 Introduction
Resprouting woody species are distributed worldwide among tropical, temperate, and Mediterranean forests. Resprouting is the dominant regeneration strategy in areas of low productivity or frequent disturbances (Del Tredici 2001; Bond and Midgley 2001; Bellingham and Sparrow 2009), such as Mediterranean landscapes where natural and anthropogenic perturbations have historically shaped vegetation and modes of ecosystem regeneration (see references in Valbuena-Carabaña et al. 2010). Although resprouting constitutes an important mechanism of natural ecosystem regeneration (Dietze and Clark 2008), in the general frame of plant ecology, the study of vegetative regeneration has been frequently disregarded in favor of sexual recruitment (Del Tredici 2001; Bond and Midgley 2001; Dietze and Clark 2008). In ecophysiological studies, trees have been traditionally considered as discrete individuals regenerating from seeds. Nevertheless, recent studies challenge this assumption as the processes of root grafting and resprouting can result in trees with multiple stems that share resources and function physiologically as a unit (Fraser et al. 2006; Tarroux et al. 2010; Baret and DesRochers 2011). Technical difficulties in the study of belowground structures have limited our understanding of root system dynamics, their relationships with aboveground structures, and their implications for ecosystem regeneration in clonal resprouting species.
Root respiration (R R) (the list of acronyms is presented in Table 1) is an important component of the carbon budget of trees (Ryan et al. 2004) and contributes to soil CO2 efflux (F S) (Hanson et al. 2000; Rey et al. 2002; Tang and Baldocchi 2005), which constitutes the largest contributor to total ecosystem respiration (Reichstein et al. 2002; Guidolotti et al. 2013). R R can be fueled by either recently assimilated (Högberg et al. 2001) or stored (Aubrey et al. 2012) carbon. The non-structural carbohydrate pool is particularly important for root growth and maintenance in resprouting species. In fact, non-structural carbohydrate pools stored in root systems of resprouters are directly related to resprouting ability and initial growth (Drake et al. 2009; Zhu et al. 2012) and have been shown to be much larger in resprouters than in non-sprouting species (Bond and Midgley 2001 and references therein). A greater amount of parenchyma and more carbohydrate storage may lead to higher maintenance respiration costs in root systems of vegetatively regenerated trees relative to sexually originated ones. In coppiced stands, periodic removal of all or part of aboveground biomass—while underground biomass remains preserved—initiates an imbalance in root/shoot biomass that may persist over time (DesRochers and Lieffers 2001; Landhäusser and Lieffers 2002; Corcuera et al. 2006; Bravo et al. 2008; Drake et al. 2009). Since fine-root shedding likely occurs after coppicing in relation to lower transpiration needs, persistence of coarse roots would contribute to a greater extent to this disequilibrium. Assuming clonal size as a proxy of clonal age (Steinger et al. 1996; Wesche et al. 2005), older and larger clones subjected to more coppicing events would be constrained by a larger imbalance. For instance, the root biomass left after a stem is felled in a clonal tree can be partially maintained by connected stems for several years, as has been shown in Populus tremuloides (DesRochers and Lieffers 2001; Jelínková et al. 2009). Therefore, an increasing disequilibrium may arise over time if the foliage that develops after repeated coppicing barely compensates for the disproportionately large amount of carbon consumed by the intact coarse root system (Iwasa and Kubo 1997; Landhäusser and Lieffers 2002). The general stagnation in growth observed worldwide in many overaged coppices after abandonment could be related to the high demand for carbohydrates by the root system, which draws resources from aboveground tree parts that would otherwise be used for stem growth and seed yield. This might be the case of Quercus pyrenaica Willd., a marcescent, root-resprouting species distributed in siliceous sub-Mediterranean mountain ranges over southwestern France, the Iberian Peninsula, and northern Morocco. Woodlands of Q. pyrenaica have been historically subject to short turns (7–15 years) of coppicing to obtain charcoal, firewood, and woody pastures. Currently, most of these coppices are abandoned due to the general rural exodus and the transition away from wood as a primary energy source that has occurred since the middle of the twentieth century. Repeated coppicing of these woodlands likely produced large clonal assemblies within stands (Valbuena-Carabaña and Gil 2013). The resulting large root-to-shoot ratio due to heavy coppicing may cause an unbalanced respiratory demand for carbohydrates in the root system which could contribute to the current degraded state of many Q. pyrenaica stands as evidenced by low productivity, high mortality rates, stem top drying, and scarce acorn yield (Cañellas et al. 2004; Bravo et al. 2008; Salomón et al. 2013).
Measuring R R presents a number of difficulties. It has been demonstrated that CO2 originating from respiration of woody tissues can diffuse radially to the atmosphere and/or dissolve in sap and move upwards in the transpiration stream (e.g., Aubrey and Teskey 2009; Cerasoli et al. 2009; Bloemen et al. 2014). Thus, the internal flux of CO2 through xylem (F T) may be responsible for large inconsistencies found in rates of woody tissue respiration, classically estimated by measurements of radial CO2 flux (Teskey and McGuire 2002). It was observed that a substantial portion of locally respired CO2 in woody tissues was transported upward instead of diffusing to the atmosphere, e.g., 35 % for tropical trees (Angert et al. 2012); 45–55, 14, and 15 % for sycamore, sweetgum, and beech trees, respectively (McGuire and Teskey 2004; Teskey and McGuire 2007); and 11 % for rimu trees (Bowman et al. 2005). Moreover, Teskey and McGuire (2007) suggested that a large portion of CO2 dissolved in tree xylem could originate in the root system and that large among-tree variability in [CO2] at the base of the stem might be explained by root size. Using different techniques such as carbon isotopes, xylem [CO2] monitoring, and stem girdling, it has been demonstrated that the autotrophic (root) component of belowground respiration is underestimated by F S measurements by up to 50 %, because root-respired CO2 dissolves in xylem sap and moves upward to the stem and leaves (Aubrey and Teskey 2009; Grossiord et al. 2012; Bloemen et al. 2014). Aubrey and Teskey (2009) estimated that, in a Populus deltoides plantation, the amount of root-respired CO2 transported aboveground via the xylem stream was twice that diffusing to the soil atmosphere. The authors calculated that only a small portion (7.8 %) of F T at the base of the stem resulted from root uptake of CO2 dissolved in soil water. Soil [CO2] is much lower than xylem [CO2] at the base of the stem (Teskey and McGuire 2007; Aubrey and Teskey 2009), suggesting that most movement of CO2 is in the direction from root to soil along the concentration gradient. Therefore, under the same soil structure and environmental conditions, differences in the relative contribution of soil water CO2 to F T among trees are likely negligible. Thus, measurements of F T at the base of the stem could be used as a proportional indicator of R R activity (Teskey and McGuire 2007; Aubrey and Teskey 2009) for comparison among different stems of the same species under the same conditions. This recently developed approach avoids the pitfalls of classic measurements of radial CO2 fluxes from roots, which are methodologically complicated by root inaccessibility and perturbations, or measurements of F S, which are controversial due to the difficulty of discriminating between heterotrophic and autotrophic respiration and their high spatial variability (Hanson et al. 2000).
Recently, the effect of clonal clump characteristics on stem growth of Q. pyrenaica was evaluated: stems belonging to large-biomass clones (characterized by large spatial extent and high number of within-clone stems and stumps) exhibited slower growth than those belonging to small-biomass clones (Salomón et al. 2013). Reduced stem growth in large clones might be conditioned by high R R, which consumes resources otherwise available for stem growth, flower production, and fruiting. To address this hypothesis, genetic and physiological approaches were integrated in this experiment: (i) genetic analyses were used to assess clonal membership since this species does not form clearly visible clonal assemblies (Valbuena-Carabaña and Gil 2013), and (ii) continuous measurements of F T were used as a proportional index to compare R R in dominant similar-sized Q. pyrenaica stems belonging to clones of differing size. Additionally, F S was measured to estimate F T contribution to R R across different clonal sizes. We predicted that large clones would have higher F T per stem and F S than small clones due to respiration of a root system disproportionate to stem size.
2 Materials and methods
2.1 Experimental site
The study was performed in an experimental plot located in the Matas de Valsaín public woodland, in the Guadarrama mountain range located in central Spain. This monospecific Q. pyrenaica forest was historically managed as a coppice since at least the twelfth century (Manuel Valdés and Rojo y Alboreca 1993) and abandoned after 1970. The 1-ha study plot represents a one-storied stand of Q. pyrenaica (781 stems ha−1) with 45-year-old stems. Site annual rainfall is 885 mm, average temperature is 10.5 °C, and soil type is humic cambisol. Climatic conditions were monitored by a weather station located 2.1 km away from the plot. Soil water content was recorded using a dielectric aquameter sensor (ECH2O; Decagon Devices, Inc.) inserted at 15 cm in depth.
2.2 Clonal assignment
Prior to the start of physiological measurements, the clonal membership of all stems in the plot was elucidated by performing genetic analyses in a hierarchical manner by gradually increasing sampling density. At the end, 541 stems were analyzed to delimit clonal clumps. The total genomic DNA was isolated from dry leaves collected in 2009 using an Invitek kit (Invisorb Spin Plant Mini Kit). Genetic analyses were made using seven nuclear microsatellite markers (QpZAG9, QpZAG36, QpZAG110, MSQ4, MSQ13, QrZAG11, and QrZAG39, developed by Dow et al. 1995, Steinkellner et al. 1997, and Kampfer et al. 2004) scored by PCR in a thermal cycler (GeneAmp® PCR System 9700; Applied Biosystems, Foster City, CA, USA) following conditions described in Valbuena-Carabaña et al. (2007). Electrophoresis and scoring of fragments were performed on a Li-Cor 4300 automated DNA sequencer (Li-Cor Biosciences, Lincoln, NE, USA) using Saga GT software (Li-Cor Biosciences, Lincoln, NE, USA) with commercial (SequaMark™; Invitrogen, Carlsbad, CA, USA) and specific internal standards. Multilocus genotypes were used to assign clonal membership of stems using GeneClone software (Arnaud-Haond and Belkhir 2007), based on the probability of occurrence of a given genotype at the observed frequency in the sample (which depends on the allele frequencies) and on the likelihood that all identical replicates were actually clones (which also depends on the sample sizes). The probabilities of the clonal stems to have a sexual origin were extremely low (P sex ranging from 2.31 · 10−19 to 1.08 · 10−205); thus, clonal assignment was performed with high statistical confidence. Clonal groups were defined as polygons whose stems had identical genotypes, including non-genotyped stems located among them.
Four clones of contrasting surface extension and stem number were selected and classified as large or small clones (two of each size class). Four even-sized and even-aged stems within each clone were selected for measurement, for a total of 16 stems. Figure 1 shows the spatial distribution of selected clones and the location of measured stems. Stem and clonal features are shown in Tables 2 and 3, respectively. Clonal and stem growth was obtained from Salomón et al. (2013) wherein two wood cores per stem were used to measure the average normal section increment during the last 10-year growing period.
2.3 Flux of root-derived CO2 through xylem (F T)
To calculate F T, measurements of sap flux and dissolved CO2 in xylem sap were needed. Sap flux density (L cm−2 sapwood s−1) was measured using constant heat thermal dissipation probes operated similar to those originally designed by Granier (1985). Pairs of thermocouples were inserted at 20 mm deep into each stem at breast height and separated at 100 mm vertically. Sap flux density was calculated from the temperature difference between the upper and lower thermocouple. Zero flow was calculated daily from the maximum temperature difference between 0400 and 0600 hours. Two pairs of probes were placed on each stem on opposite sides. Data from the two pairs were averaged to account for circumferential non-uniformity of sap flow. Calibration parameters developed by Sun et al. (2011) were applied to improve the accuracy of the measurements according to the ring porous xylem anatomy of the Q. pyrenaica stems. Sap flux (F H2O, L day−1) for each stem was calculated by multiplying 15-min average sap flux density integrated over 24 h by sapwood area. Sapwood area was determined from the color change between heartwood and sapwood of stem cores.
Xylem [CO2] was measured by inserting a solid-state non-dispersive infrared (NDIR) CO2 sensor (model GMM221; Vaisala, Helsinki, Finland) into the stem 10 cm aboveground level. Holes of 40 mm in length and 25 mm in diameter were drilled to place the sensor, which measures gaseous [CO2] (%) in equilibrium with CO2 dissolved in xylem sap ([CO2 *]). NDIR sensors were isolated from the external atmosphere with rubber sealant. Gas concentration measured at the base of the stem was converted to dissolved CO2 ([CO2 *]base, mmol L−1) by applying Henry’s law (McGuire and Teskey 2002). For this calculation, xylem temperature and sap pH were measured. A type T thermocouple was inserted in the xylem 50 mm away from the CO2 sensor to measure temperature. pH of sap expressed from twigs was determined using a portable pH meter (model 25+; Crison, Barcelona, Spain) and a pH electrode (model 52 07; Crison, Barcelona, Spain). The scarce published data regarding sap pH indicates that pH remains relatively constant within a diel period but can fluctuate during the growing season, which would affect calculations of internal [CO2 *] (Aubrey et al. 2011; Erda et al. 2014). Therefore, we measured pH every 20–25 days at peak sap flow, when F T reaches maximum values, and interpolated daily pH values between measurements. For detailed information on F T measurement methodology, see Aubrey and Teskey (2009). Xylem temperature, xylem [CO2], and sap flux density were measured every minute, averaged every 15 min, and recorded with a data logger (model CR23X; Campbell Scientific, Barcelona, Spain). Data recorded at 15-min intervals were scaled to daily total F H2O and daily average [CO2 *]base. F T (mmol day−1) was integrated over 24 h as the product of 15-min averages of F H2O and [CO2 *]base. Data recorded for individual stems were averaged per clone. Measurements were conducted in 2012 and lasted from day of year (DOY) 101 until DOY 200.
2.4 Soil CO2 efflux measurements (F S)
Ten large clones (≥9 stems and 30 m2) and ten small clones (≤5 stems and 15 m2) (Fig. 1) were selected for measurements of F S on DOY 198 of 2014. In each clone, three soil collars made of PVC, 10 cm in diameter and 4.5 cm in length, were inserted 1.5 cm into the soil at random locations 1 week before measurements. Additionally, F S was measured in six collars located in an excavated (in autumn 2013) and refilled (in spring 2014) soil area where roots and herbaceous ground cover had been removed to eliminate the autotrophic (i.e., root) respiratory component of F S. These measurements of root-free soil CO2 efflux were assumed to be equal to belowground heterotrophic respiration. F S was measured with a portable infrared gas analyzer (LI-6400; Li-Cor, Inc., Lincoln, NE, USA) and a soil chamber (LI-6400-09). Measurements of gas exchange between soil and chamber were made in closed configuration by attaching the chamber to each collar, reducing CO2 concentration inside the chamber, and then letting it increase to an upper concentration limit. These limits varied depending on efflux rate and ambient CO2 concentration. Measurements were made at ambient [CO2] and humidity across three consecutive cycles per collar. Each collar was sampled twice, at early morning and afternoon, to calculate the temperature coefficient (Q 10) of efflux, as in Zaragoza-Castells et al. (2007). From mean Q 10, F S was estimated at 16 °C, the mean soil temperature during F T monitoring (F S16, μmol m−2 s−1). We calculated this estimate based on the relationship between soil temperature and air temperature (R 2 = 0.7345, p < 0.001) and the average air temperature from DOY 146 to 200 of 2012, when substantial transpiration occurred (Fig. 2), to provide a means to compare the single-day measurements of F S with season-long measurements of F T. F S16 was partitioned into autotrophic and heterotrophic components as in Rey et al. (2002). The contribution of soil organic matter decomposition by heterotrophs (belowground heterotrophic respiration, R H16) to F S16 was obtained from the ratio root-free F S16/root F S16. The contribution of aboveground litter decomposition (R L16) was assumed to be 50 % of the remaining F S16–R H16, based on measurements in a Mediterranean coppice of Quercus cerris (Rey et al. 2002). The portion of root-respired CO2 that diffused through soil to the atmosphere (F S16-root) was calculated as
Seasonal patterns of daily mean air temperature and daily total precipitation (a), daily total sap flux (F H2O) (b), and mean dissolved [CO2 *] in sap solution at stem base ([CO2 *]base) (c) in a coppiced Quercus pyrenaica stand measured during the growing season in 2012 in two large and two small clones. Daily total sap flux and [CO2 *]base are the mean of four sampled stems per clone (three stems in clone SMALL1). Vertical dashed line on DOY 145 shows the first day on which substantial F H2O was observed. Beginning on this day, daily F H2O and [CO2 *]base were considered for comparison among clonal sizes
Additionally, to express F T and F S16-root in the same units (mmol CO2 day−1 stem−1) for comparison, F S16-root (on surface area basis) was multiplied by the clonal extension (m2) and divided by the number of stems within the clone (Table 3).
2.5 Data analysis
Since F H2O (and F T) was negligible before budburst, only data from DOY 146 to 200 (Fig. 2) was used to calculate daily average of [CO2 *]base and daily accumulated F H2O and F T per stem. Within-clone variation in [CO2 *]base, F H2O, and F T was examined by performing multiple comparisons of daily values (Tukey’s test). Differences between the two clone sizes in F H2O, [CO2 *]base, and F T were compared with hierarchical mixed models performed in R using the lme function in the nlme library (Pinheiro et al. 2014). Clone size (n = 2) was treated as a fixed factor, whereas individual clone (n = 4) was treated as a random factor. Logarithmic transformation was made to satisfy Shapiro-Wilk normality tests. To assess variability in F S16 (on area basis) and F S16-root (per stem) with clonal size (as a qualitative variable, n = 2) and clonal extension (as a quantitative variable, m2), mixed models were performed and clone was treated as a random factor (n = 20). Additionally, to assess relationships between clonal extension and clonal average F H2O, [CO2 *]base, F T, F S16, and F S16-root, we performed linear regressions to provide R 2 as an estimate of the variance explained by the model and compare magnitudes between root-respired CO2 transported in the xylem (F T) and diffused through soil to the atmosphere (F S16-root). All values presented in the text are mean (SE).
3 Results
The average temperature during the experiment was 14.7 (0.7) °C. Temperature reached its minimum of −1.9 °C on DOY 108 and its maximum of 35.2 °C on DOY 178. Total rainfall from DOY 101 to 200 was 157 mm, of which 90 % occurred before DOY 143 (Fig. 2(a)). Soil water content ranged from 17.5 % on DOY 175 (sensor installation) to 9.2 % on DOY 200. Average sap pH to calculate [CO2 *]base was 6.43 (0.03). Budburst took place ca. between DOY 137 and 145, when a substantial increase in F H2O was registered (Fig. 2(b)). STEM9 had visual symptoms of wilting, remarkably low levels of F H2O and high [CO2 *]base (Fig. 3). F H2O in STEM9 was 4.1 times lower than the lower quartile of the data distribution, and so, according to the standard boxplot rule, STEM9 was considered an outlier and excluded from statistical analyses to avoid bias in clone SMALL1.
Mean daily sap flux (F H2O), CO2 flux through xylem (F T), and dissolved CO2 concentration at stem base ([CO2 *]base) of 16 stems belonging to two large and two small clones in a coppiced Quercus pyrenaica stand measured during the growing season of 2012. Since F H2O and F T were insubstantial before and during budburst (DOY 101–145), data from DOY 146 to 200 were used to calculate the means. Different letters indicate significant differences within clone for [CO2 *]base (italic), F H2O (capital), and F T (lowercase) (Tukey’s test at p < 0.05)
In general terms, before budburst, daily mean [CO2 *]base was high and positively correlated to daily mean temperature (R 2 = 0.688, p < 0.001) and showed no relationship with daily F H2O (p = 0.420). After budburst, [CO2 *]base relationships with both temperature (R 2 = 0.397, p = 0.004) and FH2O (R 2 = 0.428, p = 0.002) were positive. Overall daily mean (from DOY 146 to 200) per-stem [CO2 *]base, F H2O, and F T were 0.27 (0.07) mmol CO2 L−1, 26.66 (2.02) L day−1, and 8.21 (2.89) mmol CO2 day−1, respectively. F H2O was not affected by clonal size (p = 0.308) (Table 4, Fig. 2(b)). However, stems of large clones had higher [CO2 *]base than stems of small clones at α = 0.10 significance level (p = 0.084) (Table 4, Fig. 2(c)). Stems belonging to large clones transported larger amounts of CO2 dissolved in the sap than stems of small clones; there were significant differences in F T among clonal sizes at α = 0.10 (p = 0.095) (Table 4). F H2O made only a small contribution to the differences in F T among clonal sizes, which were affected to a greater extent by [CO2 *]base (see Tukey’s test similarities between [CO2 *]base and F T, Fig. 3). Differences in [CO2 *]base and F T were found not only among clones, but also among stems within the same clone (Fig. 3). Linear regressions between clonal extension and clonal average F H2O, [CO2 *]base, and F T lead to the same conclusions: [CO2 *]base and F T were directly related to clonal extension (R 2 = 0.849, p = 0.079, and R 2 = 0.838, p = 0.085, respectively), whereas F H2O was not (p = 0.219) (Fig. 4).
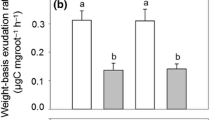
Linear regressions between clonal extension and clonal average sap flux (FH2O, L day−1) (a), [CO2] dissolved at stem base ([CO2 *]base, mmol CO2 L−1) (b), and CO2 flux through the xylem (F T, mmol CO2 day−1) (c) in two large and two small Quercus pyrenaica clones measured during the growing season of 2012. Soil CO2 efflux on surface basis (F S16, μmol m−2 s−1) (d) and root-respired CO2 diffused through soil to the atmosphere per stem (F S16-root, mmol CO2 day−1) (e) in 20 Q. pyrenaica clones measured during the growing season of 2014. [CO2 *]base, F T, F S16, and F S16-root were log transformed to satisfy Shapiro-Wilk normality tests. Statistics (R 2 and p values) apply to transformed regressions. Back-transformed values were used to display (geometric) means, SE intervals, and regression lines. Only significant (p < 0.1) regression lines are depicted
Mean F S16 and its Q 10 were 6.91 (0.31) μmol m−2 s−1 and 1.44, respectively. Given that the heterotrophic component of belowground respiration (R H16) measured in the root-free soil was 4.42 (0.75) μmol m−2 s−1, average F S16-root estimated per stem was 333.47 (53.69) mmol day−1. F S16 and F S16-root were positively related to clonal extension (p = 0.027 and p = 0.023, respectively, n = 20) (Table 4, Fig. 4). However, when clonal size as a qualitative variable was considered, F S16 and F S16-root were not significantly different among large and small clones (p = 0.312 and p = 0.247, respectively). Since F S was not measured in SMALL1 and SMALL2 (which were root trenched or harvested prior to F S measurements), the comparison between F S16-root and F T was assessed from the relationships of F S16-root and F T with clonal extension (Fig. 4(c, e)). The average ratio (%) between F T and F S16-root per stem over the sampled range of clonal extension (from 6 to 155 m2) was 1.8 %. Similarly, the F T/F S16-root ratio of the mean measured values was 2.5 %; i.e., root-respired CO2 flux through xylem was ca. 2 % of the root-respired CO2 that diffused to the atmosphere through soil (Table 5).
Mean stem diameter increment of large clones was lower compared to that of small clones (2.92 and 3.14 cm2 year−1 for large clones and 4.27 and 6.25 cm2 year−1 for small clones; Table 3). Relationships between individual stem growth and stem F H2O, [CO2 *]base, and F T were not significant (data not shown).
4 Discussion
Two methodologies were combined to understand root carbon flux in a clonal-sprouting species and its possible importance to stand degradation. First, genetic analyses provided information on the origin of the stems, and second, F T and F S were measured to compare root respiration among clonal sizes. This integrative approach provided experimental support for our hypotheses: different clonal sizes exhibited different F T and F S16-root, indicating that larger clones have higher respiration per stem than smaller clones. Moreover, in contrast to other studies, the proportion of root-respired CO2 transported through xylem (F T) was low compared to root-respired CO2 diffused through soil in our experimental system (2 %), suggesting that there will be large differences in the F T contribution to root respiration depending on species and/or site.
4.1 F T as a proportional index of root respiration
Measurements of F S are often used to elucidate R R. However, unambiguous discrimination of autotrophic (F S-root) and heterotrophic (R H) components of F S, together with F T measurements, is necessary to accurately estimate R R (R R = F S-root + F T) (Hanson et al. 2000; Aubrey and Teskey 2009). Considering the same species under the same plot conditions, we assumed that values of F T were proportional to total R R. In a poplar plantation, Aubrey and Teskey (2009) estimated that F T was approximately 66 % of total R R. In a study on Quercus robur, at high transpiration rates, the relative contribution of the autotrophic component of belowground respiration increased from 25 to 45.4 % when F T was considered (Bloemen et al. 2014). From those data, it can be inferred that 45 % [i.e., (45.4–25)/45.4] of R R was transported in the xylem stream. However, over a 5-day period, which included periods of lower transpiration, the calculated F T portion of R R decreased to 18 % [(32.8–27)/32.8]. Similarly, carbon isotope techniques applied in a Eucalyptus plantation showed that there was a reduction in the F S-root component of F S during the day, which was attributed to diversion of respired CO2 to F T, estimated to be 17 % of R R on a daily basis and 24 % at high transpiration rates (Grossiord et al. 2012). Compared to these observations in temperate and tropical trees, the magnitude of F T relative to F S-root in Q. pyrenaica was much lower (2 %).
The estimated R H16 contribution to F S16 was 64 % (i.e., 4.42 to 6.91 μmol m−2 s−1), slightly higher than the 55 and 59 % reported for a Q. cerris coppice and a Quercus douglasii parkland in the dry season (Rey et al. 2002; Tang and Baldocchi 2005). Assuming a similar contribution of R L and F S-root to F S as in the Q. cerris Mediterranean coppice, the proportion of root-respired CO2 transported through xylem (F T) relative to the root-respired CO2 that diffused through soil to the atmosphere (F S-root) was 2 %. We suspect that this upscaling underestimates the magnitude of F T compared to F S16-root because of the drier conditions in 2012 (when F T was measured) than in 2014 (when F S was measured), since F S increases with soil water availability in drought-prone regions (Reichstein et al. 2002; Rey et al. 2002; Tang and Baldocchi 2005; Guidolotti et al. 2013). In any case, F T accounted for a low proportion of R R in Q. pyrenaica. This observation highlights large variability of F T among species. In Q. pyrenaica, measured values of [CO2 *] in sap solution, ranging from 0.06 to 0.93 mmol L−1 (Fig. 3), were lower than those previously reported for several species, applying this methodology (Table 1 in Teskey et al. 2008). Nevertheless, this is the first time that [CO2 *] was measured in the Mediterranean region during summer drought (note that the study of Cerasoli et al. (2009) was performed during autumn). Summer drought in Mediterranean climates restrains respiratory processes at the ecosystem (Reichstein et al. 2002; Guidolotti et al. 2013; Rambal et al. 2014), soil (Rey et al. 2002; Tang and Baldocchi 2005), stem, and foliage levels (Maseyk et al. 2008; Rodríguez-Calcerrada et al. 2014) and may be the cause of the comparatively low [CO2 *] and F T measured in this study. Moreover, lower root water content caused by limited soil water availability would enhance F S-root to the detriment of F T due to reduced resistance to radial CO2 diffusion (Steppe et al. 2007). Accordingly, [CO2 *] as low as 0.3 mmol L−1 was observed for Q. robur in the only [CO2 *]-drought experiment existent to date after 9 days without watering, with a sharp increase in [CO2 *] once watering resumed (Saveyn et al. 2007), as was seen in this species after first autumn rain.
4.2 Relationship between F T, F S, and clonal structure
Differences in F T and [CO2 *]base between clonal sizes were significant at α = 0.10. Considering the low sample size for F T (two clones per clonal size), the α = 0.10 significance level was considered reasonable to detect actual differences. In this regard, it is worth noting that monitoring 16 stems simultaneously over 100 days represents the largest investment in the study of F T to date. Soil CO2 effluxes (F S16 and F S16-root) showed similar relationships with clonal structure. Clonal extension and F S16 and F S16-root were positively related (Table 4, Fig. 4), despite no differences among large and small clones when clonal size was considered as a qualitative variable (n = 2), likely due to the large range of extension of the ten large clones. Therefore, larger clones showed higher [CO2 *]base, F T, F S16, and F S16-root than smaller clones (Table 4, Fig. 4), which suggests higher consumption of carbohydrates for root metabolism with increasing clonal size. Although F T and stem growth were not related in this study, likely due to the reduced sample size, higher root respiratory costs per stem in larger clones could partially explain lower stem diameter growth relative to stems of smaller clones reported in temperate and Mediterranean coppices (Tanentzap et al. 2012; Salomón et al. 2013). Similarly in this study, despite even-sized and even-aged sampled stems, mean stem diameter growth increment over the last 10-year period was lower for stems in larger clones compared with smaller clones [4.44 (0.31) and 5.26 (0.72) cm2 year−1, respectively]. This difference in growth might be explained by higher initial growth of stems in larger clones (Zhu et al. 2012) followed by a greater rate of decline after 15–20 years (Corcuera et al. 2006) compared with stems in smaller clones. The root-to-shoot biomass (R/S) ratio is higher in clonal tree species compared to non-sprouters (Bond and Midgley 2001 and references therein). For instance, in oak species, a review of R/S measurements reported median values of 0.3 in temperate forests (Table 2 in Mokany et al. 2006), whereas R/S of resprouting Quercus coccifera and Quercus aquifolioides showed average values of 3.5 and 2.2, respectively (Cañellas and San Miguel 2000; Zhu et al. 2012). The ratio of aerial to belowground biomass can approach unity along broad ranges of stem size in Quercus ilex (e.g., Rambal et al. 2014). However, as clonal extension increases in Q. ilex, the relationship deviates from an isometric one due to the development of massive root systems (Canadell and Roda 1991). Assuming that clonal extension increases with age (Steinger et al. 1996; Wesche et al. 2005), the larger F T, larger F S, lower stem growth, and higher R/S ratio in large clones reinforce the hypothesis that age-related growth decline is caused partly by increased carbon allocation to storage in roots in old trees (see Ryan et al. 2004; Genet et al. 2010; Sala et al. 2012). In the particular case of old abandoned coppices, a disproportionately large energetic demand to maintain living parenchyma in the root system could become a burden because the means of carbohydrate production has been repeatedly removed (Landhäusser and Lieffers 2002). The strong root sink for carbohydrates may reduce not only aboveground growth but also flowering and fruiting, and the large carbohydrate demand would increase with increasing clonal age and size (Iwasa and Kubo 1997) in clones subjected to more coppicing events. Therefore, this physiological imbalance between root demand and shoot production should be considered as a potential cause of the degraded state of abandoned coppiced stands in Mediterranean regions, such as Q. pyrenaica stands in the Iberian Peninsula (Corcuera et al. 2006; Bravo et al. 2008).
It is worth noting that the hypothetical carbon disequilibrium holds at both stem and clone scales. When the average F T of the four stems sampled in large clones was extrapolated to the rest of their stems, accumulated F T increased to 469 and 224 mmol CO2 day−1 in LARGE1 and LARGE2, respectively, whereas in the small clones, the F T sum was 17 and 11 mmol CO2 day−1. Since the stem density within the clone (stem number/clonal extension) decreased with clonal extension (p = 0.003), differences in F S16-root between large and small clones increased when F S16-root was expressed on a stem basis (multiplying by the clonal surface and dividing by the stem number, data not shown), suggesting higher root biomass supported per stem. However, we must be cautious with these extrapolations because high intra-clonal variability was found in F T and F S16-root (Fig. 4(c, e)), suggesting an irregular distribution of living root biomass within clones, and because stem diameter was variable in the large clones. In this regard, high intra-clonal variability in F T, mainly caused by differences in [CO2 *]base (Fig. 3), may be explained by the particular interconnection of roots to stems within the clone. Stems closely connected to a large amount of live root biomass might exhibit higher [CO2 *] at the base of the stem than those with fewer connections to live root biomass. It has been seen in P. tremuloides that stump roots with connections to living stems could remain alive and functional for 70 years (DesRochers and Lieffers 2001). A similar phenomenon was observed at our experimental site, in which one excavated clone composed of eight stems and more than 50 old stumps was interconnected by more than 200 living root grafts and parental roots (R. Salomón, personal observation). This observation highlights the structural complexity that might develop in root systems of resprouting coppiced forests. For instance, this particular stand has been subjected to coppicing since at least the twelfth century (Manuel Valdés and Rojo y Alboreca 1993) and clonal extension exceeds 100 m2 in some cases (Fig. 1).
The carbon imbalance suggested by our results has silvicultural implications. Management of oak coppices is one of the largest problems currently facing Mediterranean silviculture (Cañellas et al. 2004; Montes et al. 2004). The increasing interest in implementing new uses for these abandoned stands justifies the urgent need to study them. These systems of root-resprouting clonal species present unique challenges to the application of ecophysiological and silvicultural studies. The main problems result from a lack of knowledge about what constitutes a functional unit in these ecosystems, and the technical difficulties of surveying the belowground attributes of individuals. Thinning has been advised for conversion of abandoned oak coppices into high forest, as it can enhance growth of residual trees, improve stand structure, and reduce excessive stand density (Montes et al. 2004; Bravo et al. 2008; Rodríguez-Calcerrada et al. 2011). However, thinning multi-stemmed trees could also intensify structural and physiological imbalances between shoots and roots. The reduction of stem density would result in a decrease of carbon input, whereas, for an unknown amount of time, root biomass would remain unaffected and continue to consume resources in respiratory processes. Similarly, a thinning experiment with jack pine (Pinus banksiana) revealed that the positive growth response of living residual trees connected by grafts to the root systems of removed trees was lower than that of non-grafted trees within the plot, suggesting that living residual trees were supporting roots and stumps of removed trees at the cost of their own growth (Tarroux et al. 2010). In the same way, since stems in coppiced stands are connected by the parental root system, their response to thinning could differ from that expected in classic silviculture in non-clonal stands, which could explain the limited success of thinning in Mediterranean Quercus stands to date (Valbuena-Carabaña and Gil 2013).
5 Conclusions
This work highlights the importance of clonal structure in research on root respiration and tree carbon budgets of resprouting species. The low and constant magnitude of F T compared to F S16-root (2 %) emphasizes the large variability of the contribution of F T to R R depending on species or site. On the other hand, in a formerly coppiced stand of Q. pyrenaica, large clones showed higher F T and F S16-root per stem than small clones (Table 4, Fig. 4), pointing to a direct relationship between clonal extension and the carbohydrate demands of roots. These results suggest an increasing physiological imbalance between the shoot and the root system with increasing clonal size. The genetic analyses we performed allowed identification of individual clones and provided more specific information about factors causing constrained stem growth and the general observed decay of abandoned coppiced oak stands in Mediterranean environments. High intra-clonal variability implies that in some clonal species, belowground attributes cannot be estimated from allometric relationships with aboveground features, such as stem diameter. Clonal structure after centuries of coppicing and the subsequent root biomass distribution within clones are commonly overlooked factors, although they may have important effects on respiratory carbon losses, physiological performance of individual stems, and stand dynamics.
References
Angert A, Muhr J, Negron Juarez R et al (2012) Internal respiration of Amazon tree stems greatly exceeds external CO2 efflux. Biogeosciences Discuss 9:4979–4991. doi:10.5194/bgd-9-11443-2012
Arnaud-Haond S, Belkhir K (2007) GenClone: a computer program to analyze genotypic data, test for clonality and describe spatial clonal organization. Mol Ecol Notes 7:15–17. doi:10.1111/j.1471-8286.2006.01522.x
Aubrey DP, Boyles JG, Krysinsky LS, Teskey RO (2011) Spatial and temporal patterns of xylem sap pH derived from stems and twigs of Populus deltoides L. Environ Exp Bot 71:376–381. doi:10.1016/j.envexpbot.2011.02.006
Aubrey DP, Mortazavi B, O’Brien JJ et al (2012) Influence of repeated canopy scorching on soil CO2 efflux. For Ecol Manage 282:142–148. doi:10.1016/j.foreco.2012.06.041
Aubrey DP, Teskey RO (2009) Root-derived CO2 stream rivals soil CO2 efflux. New Phytol 184:35–40. doi:10.1111/j.1469-8137.2009.02971.x
Baret M, DesRochers A (2011) Root connections can trigger physiological responses to defoliation in nondefoliated aspen suckers. Botany 89:753–761. doi:10.1139/b11-062
Bellingham PJ, Sparrow AD (2009) Multi-stemmed trees in montane rain forests: their frequency and demography in relation to elevation, soil nutrients and disturbance. J Ecol 97:472–483. doi:10.1111/j.1365-2745.2009.01479.x
Bloemen J, Agneessens L, Van Meulebroek L et al (2014) Stem girdling affects the quantity of CO2 transported in xylem as well as CO2 efflux from soil. New Phytol 201:897–907. doi:10.1111/nph.12568
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol Evol 16:45–51. doi:10.1016/S0169-5347(00)02033-4
Bowman WP, Barbour MM, Turnbull MH et al (2005) Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol 167:815–828. doi:10.1111/j.1469-8137.2005.01478.x
Bravo JA, Roig S, Serrada R (2008) Selvicultura en montes bajos y medios de Quercus ilex L., Q. pyrenaica Willd. y Q. faginea Lam. In: Serrada R, Montero G (eds) Compend. Selvicultura Apl. en España. Instituto Nacional Investigaciones Agrarias, Madrid, pp 657–744
Canadell J, Roda F (1991) Root biomass of Quercus ilex in a montane Mediterranean forest. Can J For Res 21:1771–1778. doi:10.1139/x91-245
Cañellas I, Del Río M, Roig S, Montero G (2004) Growth response to thinning in Quercus pyrenaica Willd. coppice stands in Spanish central mountain. Ann For Sci 61:243–250. doi:10.1051/forest:2004017
Cañellas I, San Miguel A (2000) Biomass of root and shoot systems of Quercus coccifera shrublands in Eastern Spain. Ann For Sci 57:803–810. doi:10.1051/forest:2000160
Cerasoli S, McGuire MA, Faria J et al (2009) CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J Exp Bot 60:99–105. doi:10.1093/jxb/ern272
Corcuera L, Camarero JJ, Sisó S, Gil-Pelegrín E (2006) Radial-growth and wood-anatomical changes in overaged Quercus pyrenaica coppice stands: functional responses in a new Mediterranean landscape. Trees - Struct Funct 20:91–98. doi:10.1007/s00468-005-0016-4
Del Tredici P (2001) Sprouting in temperate trees: a morphological and ecological review. Bot Rev 67:121–140. doi:10.1007/BF02858075
DesRochers A, Lieffers VJ (2001) Root biomass of regenerating aspen (Populus tremuloides) stands of different densities in Alberta. Can J For Res 31:1012–1018. doi:10.1139/cjfr-31-6-1012
Dietze MC, Clark JS (2008) Changing the gap dynamics paradigm: vegetative regeneration control on forest response to disturbance. Ecol Monogr 78:331–347. doi:10.1890/07-0271.1
Dow BD, Ashley MV, Howe HF (1995) Characterization of highly variable (GA/CT)n microsatellites in the bur oak, Quercus macrocarpa. Theor Appl Genet 91:137–41. doi:10.1007/BF00220870
Drake PL, Mendham DS, White D a, Ogden GN (2009) A comparison of growth, photosynthetic capacity and water stress in Eucalyptus globulus coppice regrowth and seedlings during early development. Tree Physiol 29:663–674. doi:10.1093/treephys/tpp006
Erda FG, Bloemen J, Steppe K (2014) Quantifying the impact of daily and seasonal variation in sap pH on xylem dissolved inorganic carbon estimates in plum trees. Plant Biol 16:43–48. doi:10.1111/plb.12009
Fraser EC, Lieffers VJ, Landhäusser SM (2006) Carbohydrate transfer through root grafts to support shaded trees. Tree Physiol 26:1019–1023. doi:10.1093/treephys/26.8.1019
Genet H, Bréda N, Dufrêne E (2010) Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol 30:177–92. doi:10.1093/treephys/tpp105
Granier A (1985) Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann For Sci 42:193–200. doi:10.1051/forest:19850204
Grossiord C, Mareschal L, Epron D (2012) Transpiration alters the contribution of autotrophic and heterotrophic components of soil CO2 efflux. New Phytol 194:647–653. doi:10.1111/j.1469-8137.2012.04102.x
Guidolotti G, Rey A, D’Andrea E et al (2013) Effect of environmental variables and stand structure on ecosystem respiration components in a Mediterranean beech forest. Tree Physiol 33:960–72. doi:10.1093/treephys/tpt065
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146. doi:10.1023/A:1006244819642
Högberg P, Nordgren A, Buchmann N et al (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Lett to Nat 411:789–792. doi:10.1038/35081058
Iwasa Y, Kubo T (1997) Optimal size of storage for recovery after unpredictable disturbances. Evol Ecol 11:41–65. doi:10.1023/A:1018483429029
Jelínková H, Tremblay F, Desrochers A (2009) Molecular and dendrochronological analysis of natural root grafting in Populus tremuloides (Salicaceae). Am J Bot 96:1500–5. doi:10.3732/ajb.0800177
Kampfer S, Lexer C, Glössl J, Steinkellner H (2004) Characterization of (GA)n microsatellite loci from Quercus robur. Hereditas 129:183–186. doi:10.1111/j.1601-5223.1998.00183.x
Landhäusser SM, Lieffers VJ (2002) Leaf area renewal, root retention and carbohydrate reserves in a clonal tree species following above-ground disturbance. J Ecol 90:658–665. doi:10.1046/j.1365-2745.2002.00699.x
Manuel Valdés C, Rojo y Alboreca A (1993) Valsaín forest in the XVIII century: an example of forest management in the pre-industrial era. Investig Agrar Sist Recur 3:217–229
Maseyk K, Grünzweig JM, Rotenberg E, Yakir D (2008) Respiration acclimation contributes to high carbon-use efficiency in a seasonally dry pine forest. Glob Chang Biol 14:1553–1567. doi:10.1111/j.1365-2486.2008.01604.x
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578. doi:10.1093/treephys/24.5.571
McGuire MA, Teskey RO (2002) Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiol 22:807–811. doi:10.1093/treephys/22.11.807
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root:shoot ratios in terrestrial biomes. Glob Chang Biol 12:84–96. doi:10.1111/j.1365-2486.2005.001043.x
Montes F, Cañellas I, Del Rio M et al (2004) The effects of thinning on the structural diversity of coppice forests. Ann For Sci 61:771–779. doi:10.1051/forest:2004074 771
Pinheiro J, Bates D, DebRoy S, et al. (2014) nlme: linear and nonlinear mixed effects models. R Package, version 3.1-117
Rambal S, Lempereur M, Limousin JM et al (2014) How drought severity constrains gross primary production (GPP) and its partitioning among carbon pools in a Quercus ilex coppice? Biogeosciences 11:6855–6869. doi:10.5194/bgd-11-8673-2014
Reichstein M, Tenhunen J, Roupsard O et al (2002) Ecosystem respiration in two Mediterranean evergreen Holm Oak forests: drought effects and decomposition dynamics. Funct Ecol 16:27–39. doi:10.1046/j.0269-8463.2001.00597.x
Rey A, Pegoraro E, Tedeschi V et al (2002) Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob Chang Biol 8:851–866. doi:10.1046/j.1365-2486.2002.00521.x
Rodríguez-Calcerrada J, Martin-StPaul NK, Lempereur M et al (2014) Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agric For Meteorol 195–196:61–72. doi:10.1016/j.agrformet.2014.04.012
Rodríguez-Calcerrada J, Pérez-Ramos IM, Ourcival J-M et al (2011) Is selective thinning an adequate practice for adapting Quercus ilex coppices to climate change? Ann For Sci 68:575–585. doi:10.1007/s13595-011-0050-x
Ryan M, Binkley D, Fownes J et al (2004) An experimental test of the causes of forest growth decline with stand age. Ecol Monogr 74:393–414. doi:10.1890/03-4037
Sala A, Woodruff DR, Meinzer FC (2012) Carbon dynamics in trees: feast or famine? Tree Physiol 32:764–775. doi:10.1093/treephys/tpr143
Salomón R, Valbuena-Carabaña M, Gil L, González-Doncel I (2013) Clonal structure influences stem growth in Quercus pyrenaica Willd. coppices: bigger is less vigorous. For Ecol Manage 296:108–118. doi:10.1016/j.foreco.2013.02.011
Saveyn A, Steppe K, Lemeur R (2007) Drought and the diurnal patterns of stem CO2 efflux and xylem CO2 concentration in young oak (Quercus robur). Tree Physiol 27:365–374. doi:10.1093/treephys/27.3.365
Steinger T, Korner C, Schmid B (1996) Long-term persistence in a changing climate: DNA analysis suggests very old ages of clones of alpine Carex curvula. Oecologia 105:94–99. doi:10.1007/BF00328796
Steinkellner H, Fluch S, Turetschek E, Lexer C, Streiff R, Kremer A, Burg K, Glossl J (1997) Identification and characterization of (GA/CT)n - microsatellite loci from Quercus petraea. Plant Mol Biol 33:1093–1096. doi:10.1023/A:1005736722794
Steppe K, Saveyn A, McGuire MA et al (2007) Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct Plant Biol 34:785–792. doi:10.1071/FP07077
Sun H, Aubrey DP, Teskey RO (2011) A simple calibration improved the accuracy of the thermal dissipation technique for sap flow measurements in juvenile trees of six species. Trees - Struct Funct 26:631–640. doi:10.1007/s00468-011-0631-1
Tanentzap AJ, Mountford EP, Cooke AS, Coomes DA (2012) The more stems the merrier: advantages of multi-stemmed architecture for the demography of understorey trees in a temperate broadleaf woodland. J Ecol 100:171–183. doi:10.1111/j.1365-2745.2011.01879.x
Tang J, Baldocchi DD (2005) Spatial–temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 73:183–207. doi:10.1007/s10533-004-5889-6
Tarroux E, DesRochers A, Krause C (2010) Effect of natural root grafting on growth response of jack pine (Pinus banksiana) after commercial thinning. For Ecol Manage 260:526–535. doi:10.1016/j.foreco.2010.05.008
Teskey RO, McGuire MA (2002) Carbon dioxide transport inxylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ 25:1571–1577. doi:10.1046/j.1365-3040.2002.00961.x
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30:570–579. doi:10.1111/j.1365-3040.2007.01649.x
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32. doi:10.1111/j.1469-8137.2007.02286.x
Valbuena-Carabaña M, de Heredia UL, Fuentes-Utrilla P et al (2010) Historical and recent changes in the Spanish forests: a socio-economic process. Rev Palaeobot Palynol 162:492–506. doi:10.1016/j.revpalbo.2009.11.003
Valbuena-Carabaña M, Gil L (2013) Genetic resilience in a historically profited root sprouting oak (Quercus pyrenaica Willd.) at its southern boundary. Tree Genet Genomes 9:1129–1142. doi:10.1007/s11295-013-0614-z
Valbuena-Carabaña M, González-Martínez SC, Hardy OJ, Gil L (2007) Fine-scale spatial genetic structure in mixed oak stands with different levels of hybridization. Mol Ecol 16:1207–19. doi:10.1111/j.1365-294X.2007.03231.x
Wesche K, Ronnenberg K, Hensen I (2005) Lack of sexual reproduction within mountain steppe populations of the clonal shrub Juniperus sabina L. in semi-arid southern Mongolia. J Arid Environ 63:390–405. doi:10.1016/j.jaridenv.2005.03.014
Zaragoza-Castells J, Sánchez-Gómez D, Valladares F et al (2007) Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant Cell Environ 30:820–33. doi:10.1111/j.1365-3040.2007.01672.x
Zhu W-Z, Xiang J-S, Wang S-G, Li M-H (2012) Resprouting ability and mobile carbohydrate reserves in an oak shrubland decline with increasing elevation on the eastern edge of the Qinghai–Tibet Plateau. For Ecol Manage 278:118–126. doi:10.1016/j.foreco.2012.04.032
Acknowledgments
We are grateful to Javier Donés, director of the Centro de Montes y Aserradero de Valsaín, for his economic and logistic support. We also thank Elena Zafra and Zaida Lorenzo for their inestimable help in lab analyses and field work, a task where Matías Millerón, César Otero, Jesús Alonso, Guillermo González, Aída Rodríguez, Paula Guzmán, Mario Zabal, and Saúl Delgado also participated
Funding
This work was funded by CAM P2009/AMB-1668 project and OAPN Prop23/10 JD/pl contract. Roberto Salomón was supported by a Ph.D. scholarship from the “Universidad Politécnica de Madrid.” Jesús Rodríguez-Calcerrada was supported by a Juan de la Cierva contract from the Spanish Ministry of Economy and Competitiveness.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Erwin Dreyer
Contribution of the co-authors
Roberto Salomón, María Valbuena-Carabaña, and Jesús Rodríguez-Calcerrada: designing the experiment, running data analysis, and writing the paper
Doug Aubrey, MaryAnne McGuire, and Robert Teskey: teaching required physiological techniques to the first author and writing the paper
Luis Gil and Inés González-Doncel: supervising the work, coordinating research project, and writing the paper
Rights and permissions
About this article
Cite this article
Salomón, R., Valbuena-Carabaña, M., Rodríguez-Calcerrada, J. et al. Xylem and soil CO2 fluxes in a Quercus pyrenaica Willd. coppice: root respiration increases with clonal size. Annals of Forest Science 72, 1065–1078 (2015). https://doi.org/10.1007/s13595-015-0504-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13595-015-0504-7