Abstract
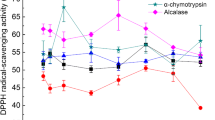
The main aim of the present study was to optimize the hydrolysis conditions of whey protein isolate digested by a protease preparation from Aspergilus oryzae through response surface method (RSM) in order to achieve the maximum angiotensin I-converting enzyme (ACE)-inhibitory activity and antioxidant properties. The effects of hydrolysis conditions including time (2, 13, 24, 35, and 46 h), temperature (40, 45, 50, 55, and 60 °C) and pH (6, 6.5, 7, 7.5, and 8) were investigated on the bioactivity of whey protein hydrolysates. Each process parameter emerged to have a dual effect on bioactivity; that is, increase in all variables promoted bioactive peptide generation through facilitating enzyme access to the primary protein sequence by partial unfolding of the compact globular assemblies of whey proteins. However, prolonged digestion at high temperatures and alkaline pH were concomitant with decreased bioactivity which are attributed to hydrolysate aggregation and splitting of bioactive peptides into biologically inactive counterparts, respectively. Nonetheless, some discrepancies were observed between the trend of ACE-inhibitory activity and that of antioxidant activity changes which was explained in light of their general characteristics. RSM efficiently identified the critical levels of each variable to obtain maximum bioactivity. It was shown that hydrolysate prepared at 56.54 °C and pH 6.04 resulting from digestion for 3.89 h exerted 74% ACE-inhibitory activity, 666.31 μM trolox equivalent/mg antioxidant activity, and 14.03% hydrolysis degree.
摘要:
本文采用响应面法对米曲霉蛋白酶水解乳清分离蛋白的条件进行优化,以取得最大的血管紧张素转化酶(ACE)抑制活性和抗氧化特性。研究了水解时间(2, 13, 24, 35 和46 h)、温度(40, 45, 50. 55 和 60 °C) 和 pH (6, 6.5, 7, 7.5 和8)对乳清蛋白水解物生物活性的影响。每个工艺参数对生物活性会产生双重影响,也就是增加所有变量,通过将致密的球状乳清蛋白部分折叠解开使酶进入一级蛋白序列,从而促进生物活性肽的产生。然而,在高温和碱性pH条件下,延长水解时间会降低水解物的生物活性,主要原因是随着水解时间的延长,水解物聚集以及生物活性肽裂解成没有生物活性的物质。虽然如此,血管紧张素转化酶(ACE)抑制性和抗氧化活性会出现不一致,这由于两者的特性不同。响应面法(RSM)能够有效地确定并获得最大生物活性的每个变量的水平。实验结果表明:在56.54 °C,pH 6.04 水解时间为3.89 h得到的水解物具有74%的血管紧张素转化酶(ACE)抑制活性,等效抗氧化活性为666.31 μM,水解度为14.03%。



Similar content being viewed by others
References
Alder-Nissen J (1986) Enzymaic hydrolysis of food proteins. Elsevier, New York
Costa EL, Gontijo JAR, Netto FM (2007) Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates. Int Dairy J 17:632–640
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20:1637–1648
Fitzsimon SM, Mulvihill DM, Morris ER (2007) Denaturation and aggregation process in thermal gelation. Food Hydrocolloid 21:638–644
Fluegel SM, Shultz TD, Powers JR, Clerk S, Barbosa-Leiker C, Wright BR, Freson TS, Fluegel HL, Minch JD, Schwarzkopf LK, Miller AJ (2010) Whey beverages decrease blood pressure in prehypertensive and hypertensive young men and women. Int Dairy J 20:753–760
Fuente MA, Singh H, Hemar Y (2002) Recent advances in the characterization of heat-induced aggregates and intermediates of whey proteins. Trends Food Sci Tech 13:262–274
Giroux H, Britten M (2004) Heat treatment of whey proteins in the presence of anionic surfactants. Food hydrocolloid 18:685–692
Guo Y, Pan D, Tanokura M (2009) Optimisation of hydrolysis conditions for the production of the angiotensin-І-converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology. Food Chem 114:328–333
Hernandez-Ledesma B, Recio I, Ramos M, Amigo L (2002) Preparation of ovine and caprine β-lactoglobulin hydrolysates with ACE-inhibitory activity: identification of active peptides from caprine β-lactoglobulin hydrolysed with thermolysin. Int Dairy J 12:805–812
Hernández-Ledesma B, Ramos M, Gómez-Ruiz JA (2011) Bioactive components of ovine and caprine cheese whey. Small Ruminant Res 101:196–204
Ju ZY, Kilara A (1998) Gelation of hydrolysates of a whey protein isolate induced by heat, protease, salts and acid. Int Dairy J 8:303–309
Kim SB, Seo IS, Khan MA, Ki KS, Nam MS, Kim HS (2007) Separation of iron-binding protein from whey through enzymatic hydrolysis. Int Dairy J 17:625–631
Korhonen H (2009) Milk-derived bioactive peptides: from science to application. J Funct Food 1:177–187
Korhonen H, Pihlanto-Lepällä A, Rantamäki P, Tupasela T (1998) Impact of processing on bioactive proteins and peptides. Trend Food Sci Tech 9:307–319
Madadlou A, Emam-Djomeh Z, Mousavi ME, Ehsani M, Javanmard M, Sheehan D (2009a) Response surface optimization of an artificial neural network for predicting the size of re-assembled casein micelles. Comput Electron Agric 68:216–221
Madadlou A, Mousavi ME, Emam-Djomeh Z, Sheehan D, Ehsani M (2009b) Alkaline pH does not disrupt re-assembled casein micelles. Food Chem 116:929–932
Madadlou A, Iacopino D, Sheehan D, Emam-Djomeh Z, Mousavi ME (2010) Enhanced thermal and ultrasonic stability of a fungal protease encapsulated within biomimetically generated silicate nanospheres. BBA-Gen Sub 1800:459–465
Madadlou A, Sheehan D, Emam-Djomeh Z, Mousavi ME (2011) Ultrasound-assisted generation of ACE-inhibitory peptides from casein hydrolyzed with nanoencapsulated protease. J Sci Food Agric 91:2112–2116
Madureira AR, Tavares T, Gomesa AM, Pintado M, Malcata F (2010) Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci 93:437–455
Mizuno S, Nishimura S, Matsuura K, Gotou T, Yamamoto N (2004) Release of short and proline-rich antihypertensive peptides from casein hydrolysate with an Aspergillus oryzae protease. J Dairy Sci 87:3183–3188
Nelson D (2005) Lehninger principles of biochemistry. Amazon, New York
Otte J, Shalabi S, Zakora M, Pripp A, El-Shabrawi S (2007) Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: effect of substrate, enzyme and time of hydrolysis. Int Dairy J 17:488–503
Peña-Ramos EA, Xiong YL (2001) Antioxidative activity of whey protein hydrolysates in a liposomal system. J Dairy Sci 84:2577–2583
Periago MJ, Vidal ML, Ros G, Rincón F, Martínez C, López G, Rodrigo J, Martínez I (1998) Influence of enzymatic treatment on the nutritional and functional properties of pea flour. Food Chem 63:71–78
Pihlanto-Leppälä A (2001) Bioactive peptides derived from bovine whey proteins: opioid and ACE-inhibitory peptides. Trends Food Sci Tech 11:347–356
Pihlanto-Leppälä A, Koskinen P, Pillola K, Tupasela T, Korhonen H (2000) Angiotensin-І-converting enzyme inhibitory properties of whey protein digest: concentration and characterisation of active peptides. J Dairy Res 67:53–64
Ranathunga S, Rajapakse KS (2006) Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur Food Res Technol 222:310–315
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med 26:1231–1237
Rho SJ, Lee JS, Chung YI, Kim YW, Lee HG (2009) Purification and identification of an angiotensin І-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem 44:490–493
Samaranayaka AGP, Li-Chen ECY (2011) Food-derived peptidic antioxidants: a review of their production, assessment, and potential application. J Funct Food 3:229–254
Sinha R, Radha C, Prakash J, Kaulm P (2007) Whey protein hydrolysate: functional properties, nutritional quality and utilization in beverage formulation. Food Chem 101:1484–1491
Smithers GW (2008) Whey and whey proteins—from gutter-to-gold. Int Dairy J 18:695–704
Ven CVD, Gruppen H, Bont BAD, Vorgan GJA (2002) Optimization of the angiotensin converting enzyme inhabitation by whey protein hydrolysates using response surface methodology. Int Dairy J 12:813–820
Acknowledgment
The authors thank the Iran National Scientific Foundation (INSF) for its financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Goudarzi, M., Madadlou, A., Mousavi, M.E. et al. Optimized preparation of ACE-inhibitory and antioxidative whey protein hydrolysate using response surface method. Dairy Sci. & Technol. 92, 641–653 (2012). https://doi.org/10.1007/s13594-012-0081-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-012-0081-6