Abstract
Residues of acaricide coumaphos were assessed in honey, bee brood, and beeswax during a 2-year field experiment. Honey, bee brood, and beeswax samples were collected before and after routine use of coumaphos in the treatment of bee colonies against varroosis in two consecutive years. Determination of coumaphos in honey and bee brood was based on RP-HPLC with UV detection after a liquid-liquid extraction with hexane or ethyl acetate. Coumaphos in beeswax was identified and quantified by GC/MS. Results indicate the undetectable presence of coumaphos in honey. In bee brood, coumaphos was observed after the treatment. In beeswax, the accumulation of coumaphos was determined not only in hives where it was used but also in hives nearby in which coumaphos was not used. Results indicate the accumulation of coumaphos in bee brood and beeswax. Due to the coumaphos accumulation this drug should be used only in strongly affected bee colonies.
Similar content being viewed by others
1 Introduction
The ectoparasitic mite Varroa destructor (Varroa) is considered as a major threat to the honeybee Apis mellifera. A varroa female goes to the larval cell before capping where it feeds on the pupae and completes its reproductive cycle. Feeding on the larval hemolymph causes weight loss, malformation, and shortens the life span of honeybees, and the mite also serves as a vector of the pathogens (Tabart et al. 2013; McDonnell et al. 2013). The Varroa infestation has a strong impact on bee colony health and the economy of beekeeping. In the untreated colonies, bee mortality could be up to 100%. Therefore, a wide array of chemical compounds, including a range of synthetic acaricides and insecticides, are widely applied on beehives to control this parasite. One of them is coumaphos (O-3-chloro-4-methyl-2-oxo-2H-chromen-7-yl O,O-diethylphosphorothioate), which is an organophosphate insecticide (Martel and Zeggane 2002; Karazafiris et al. 2008) and a stable lipophilic compound. It is widely used to control varroosis in bee colonies and for the treatment of ectoparasitoses in livestock. For the treatment of bee colonies, it is in use in strip and liquid form. Coumaphos targets cholinergic signaling through acetylcholine esterase inhibition and comprises the majority of excitatory neurotransmission in the Varroa’s nervous system (Millar and Denholm 2007). Beside anti-Varroa properties, some toxic effects of coumaphos on bees were reported as increased mortality rate in developing larvae (Berry et al. 2013; Zhu et al. 2014), reduced longevity in adult bees which were exposed to coumaphos as immatures (Wu et al. 2011; Berry et al. 2013), and higher number of queen cells under construction in treated hives (Berry et al. 2013).
Inevitably, the utilization of acaricides in apiculture has resulted in residues being frequently found in bee products. Water-soluble products are mainly identified in the honey, while fat-soluble products have a higher affinity for the beeswax. Most of the synthetic acaricides are lipophilic and therefore accumulate in beeswax, whereas residues in honey are relatively low (Karazafiris et al. 2008; Xu et al. 2009; Valdovinos-Flores et al. 2016). These chemicals can also resist the wax melting temperature; therefore, some of them can accumulate for years as it is a common beekeeping practice to recycle wax almost continuously in the form of the foundations on which bees construct a complete comb (Ravoet et al. 2015). It has been described that coumaphos was found persistent in the beeswax for 5 years (Martel et al. 2007; Zhu et al. 2014) with an estimated half-life of 115–346 days (Martel et al. 2007).
Maximum residue limits (MRL) of several veterinary formulations in honey have been fixed in many countries to reduce the health risk for consumers. According to EU regulations, MRL for coumaphos is 100 μg/kg (European Commission 2010). The contamination of honey with coumaphos is widely documented in the scientific literature. Martel et al. (2007) found coumaphos in honey in concentrations up to 2.02 mg/kg during the treatment while on the 30th day of treatment the concentration decreased to 0.05 mg/kg. Karazafiris et al. (2008) reported concentrations to be the highest (0.131 mg/kg) 35 days after coumaphos strips were placed into the hive. The same study showed that the residue levels in honey depended on the application technique and the duration of treatment. Following treatment, the presence of coumaphos in honey was found in 64% of tested hives with concentrations from 0.005 to 0.040 mg/kg (Valdovinos-Flores et al. 2016).
Although, the coumaphos toxicity on developing larvae was described in the literature (Berry et al. 2013; Zhu et al. 2014), we did not find any data about coumaphos accumulation in bee brood. Contrary, coumaphos concentrations in beeswax are well documented and were found to range from 0.155 to 2.220 mg/kg following the treatment with coumaphos (Valdovinos-Flores et al. 2016), while Berry et al. (2013) found even higher concentrations. Coumaphos was also found in samples of commercial beeswax (Serra-Bonvehí and Orantes-Bermejo 2010). In newly produced beeswax, coumaphos was found in small amounts (less than 1 μg/kg) (van Buren et al. 1992).
The aim of this study was to determine the levels of coumaphos in honey, bee brood, and beeswax before and after Varroa treatment with a commercially available drug that contains coumaphos during a 2-year field experiment and get the direct information about coumaphos accumulation in different matrices during its use as a drug.
2 Materials and methods
2.1 Varroa treatment and sample collection
Ten bee colonies were included in the experiment and were followed during two consecutive years. Five colonies were treated with a therapeutic dose of coumaphos (two strips of CheckMite; Bayer, Berlin, Germany) in August each year. The five other colonies were used as controls and were treated with formic acid at the same time. In treated beehives, the strips with coumaphos were suspended between frames in the brood chamber. According to the manufacturer’s instructions, the strips with coumaphos were left in beehives for 42 days.
Samples of honey, bee larvae, and beeswax were collected before the beginning of the treatment and 42 days later, on the day of strip removal. The same procedure was performed in the following year. Altogether, four sample collections were performed and thus 40 samples of honey, bee brood and beeswax were collected. In each hive surveyed, the third frame from a hanging strip was selected for sampling. Approximately 30 g of honey was scooped out by a plastic spoon and approximately 15 g of bee larvae were collected from capped brood cells. Additionally, a lump of approximately 10 g of wax was cut off in a single area. All samples were processed immediately after collection.
2.2 Clean-up and extraction procedure
2.2.1 Honey samples
Before analysis, honey was left dripping through a fine mesh to remove any impurities and wax particles.
For coumaphos extraction, 5 g of honey was placed into a conical 50 mL centrifuge tube (Sarstedt, Nuembrecht, Germany). Five grams of sodium sulfate and 15 mL of hexane (both produced by Merck; Darmstadt, Germany) were added. After vigorous shaking and ultrasonic bath, the samples were centrifuged for 10 min at 2500 rpm and 20°C (Hettich, Tuttlingen, Germany). The liquid phase was decanted into a new conical 15-mL centrifuge tube (Sarstedt, Nuembrecht, Germany) and evaporated to dryness under nitrogen in a water bath at 35°C. The extract was reconstituted in 1 mL of acetonitrile and filtered through a 0.45-μm organic membrane filter prior to chromatographic analysis.
2.2.2 Bee larvae samples
After the collection of bee larvae, all the impurities were removed, the material was homogenized (Ultraturax, Ika, Staufen, Germany) and frozen until analysis.
For coumaphos extraction, 5 g of bee larvae was placed into a conical centrifuge tube (50 mL, Sarstedt, Nuembrecht, Germany) and 8 g of sodium sulfate and 28 mL of hexane was added. The extraction was the same as described for honey samples.
2.2.3 Wax samples
The wax was chopped up with a scalpel and washed with distilled water. The washed wax was dried in air in a dark room and frozen until analysis. Matrix solid-phase extraction was used for determination of coumaphos in beeswax. After homogenization, 0.2 g of beeswax was mixed with 2 g of RP-8, 0.2 g of sodium sulfate (both by Merck, Darmstadt, Germany), and 20 μL of internal standard (alpha-HCH-d6) in a mortar using a pestle. For the recovery study, 20 μL of coumaphos standard (Fluka, St. Gallen, Switzerland) was also added and mixed with solid-phase extraction (SPE) material. The homogeneous powder was then transferred to the 12-mL SPE column already containing 2 g C18 material (Varian, Palo Alto, CA, USA) previously washed with 5 mL of acetonitrile. A 3-mL SPE column containing 500 mg of primary-secondary amine (PSA) (Varian, Palo Alto, CA, USA) was attached under the C18 column and was also previously washed with 5 mL of acetonitrile. Coumaphos was eluted from the tandem columns with 15 mL of acetonitrile. The eluate was evaporated under the stream of nitrogen at 40°C, and the solvent was exchanged to ethyl acetate. The final volume of the extract was 780 μL to which 20 μL of injection internal standard phenanthrene-d10 (Merck, Darmstadt, Germany) was added.
2.3 Assay procedure of coumaphos measurements
Concentrations of coumaphos in honey and bee brood was determined with an HPLC system consisted of a P2000 pump (Spectra Physics, San Jose, CA, USA), an AS3000 autosampler (Spectra Physics, San Jose, CA, USA), a degasser, binary pump, UV detector, and a SPECTRA 360 computing integrator (Spectra Physics, San Jose, CA, USA) connected to a PC. The separation was performed on a Luna column (5 μ C18(2), 100 Å, 250 × 4.6 mm, Phenomenex, Torrance, CA, USA). Acetonitrile and distilled water were used for mobile phases in relations 60:40, 60:40, 95:5, 95:5, 60:40 and 60:40 at 0, 7, 8, 17, 18 and 24 min, respectively. The measurements were performed at 289 nm UV wavelength (Figure 1).
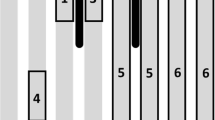
GC-MS (SIM) chromatogram for coumaphos in blank sample, matrix-matched standard solution, and wax sample. The three chosen SIM ions for coumaphos were (m/z 362, 364, 226) and the retention time was 31.634 min. A blank sample, B matrix standard solution at 0.05 mg/kg, C positive sample of beeswax (c = 0.19 mg/kg). BT-1 before treatment in the first season of experiment, AT-1 after treatment in the first season of experiment, BT-2 before treatment in the second season of experiment, AT-2 after treatment in the second season of experiment.
Samples of honey and bee brood for determining the limits of determination (LODs) and limits of quantification (LOQs), recovery, and precision were free of coumaphos. Recovery experiments were performed by spiking honey and bee brood samples (5 g) with concentrations 50, 100, 200, and 500 μg/kg of coumaphos (Sigma-Aldrich, St. Louis, MO, USA). Prior to sample analysis by this method, the spiked samples were let to stand at room temperature for 5 h to achieve the solvent evaporation and coumaphos distribution in the samples. Six of the sample duplicates were used to determine the recovery and intra-day precision. Intra-day precision was assessed over the course of 1 day and calculated as the percentage coefficient of variation (CV%) at each concentration. Recovery was assessed by comparing the determined concentration of spiked samples to the theoretical concentrations (Table I). Linearity (r 2) was determined with fortified samples containing 50, 100, 200, 500 and 1.000 μg/kg of coumaphos. r 2 for coumaphos measurements in honey and bee brood ranged between 0.9966 and 1.000 and 0.9982 and 0.9999, respectively. LOD and LOQ were calculated as 3 and 10 times, respectively, the signal-to-noise ratio of samples spiked at low concentrations. LOD and LOQ for coumaphos in honey and bee brood were 15 and 50 μg/kg.
Coumaphos in beeswax was determined by GC/MS. An AT 6890 gas chromatograph (Agilent Technologies, Wilmington, DE, USA) configured with a CIS-4 Plus PTV inlet (Gerstel, Muhlheim, Germany) and an Agilent 5975 mass-selective detector with inert ion source were used. Samples were injected with an AT 7683B autosampler, which is capable of speed-controlled injections. Programmable-temperature vaporizer (PTV) was used to inject 10 μL of extract using solvent vent mode. For the GC separations, a HP-5MSi fused silica capillary column (30 m × 0.25 mm, 0.25 μm) coated with 5% diphenylmethyl polysiloxane stationary phase (Agilent Technologies) was used with helium as the carrier gas in the constant pressure mode. The MS was operated in electron ionization (EI) mode at 70 eV. Temperatures of the transfer line, ion source, and quadrupole were set at 280, 230 and 150°C, respectively. The column temperature was held at 70°C for 2 min, programmed at 25°C/min to 150°C, then at 3°C/min to 200°C and finally at 8°C/min to 280°C, which was held for 14 min.
Quantitative analysis of pesticides was performed using selected ion monitoring (SIM). SIM ions used for quantitation were m/z 362, 364, and 226. The pesticide peaks were confirmed with retention times and abundance ratios of target ions and two qualifier ions (Figure 2). Matrix-matched calibration was used to compensate for matrix effects.
Recoveries ranged between 93 and 106%. LOQ for coumaphos in beeswax was 50 μg/kg.
2.4 Statistical analysis
SPSS version 21 (IBM, Chicago, IL, USA) commercial software was used for statistical analyses. The Shapiro-Wilk test was used for the estimation of data distribution. Results of coumaphos concentration in bee brood and beeswax were normally distributed; therefore, an independent t test was used for the evaluation of statistically significant differences between control and treated groups. Time-dependent data of coumaphos concentration in beeswax and bee brood were analyzed by repeated measures ANOVA. Additionally, one-tailed Pearson’s correlation was used to correlate beeswax coumaphos concentration from treated and non-treated hives. Concentrations below LOQ were considered as the LOQ value. All results are presented as mean ± SE, and statistical significance was considered at P <0.05.
3 Results
3.1 Coumaphos levels in honey, bee brood, and beeswax
Coumaphos levels in all honey samples from control (n = 5) and experimental (n = 5) bee colonies collected before and after treatment in two consecutive years were all found below LOQ (50 μg/kg).
In control bee colonies (n = 5), coumaphos levels in bee brood were found below LOQ (50 μg/kg). In experimental bee colonies (n = 5) before treatment, coumaphos levels in bee brood were also found below LOQ. However, after the treatment, coumaphos concentrations in bee brood from observed hives ranged in the first season of the experiment between 143 and 568 μg/kg and in the second season between 58 and 216 μg/kg. After the treatment, concentrations were significantly higher (P <0.01) in comparison to controls. Coumaphos levels in bee brood are presented in Figure 3.
Coumaphos concentration in beeswax from control (n = 5) and treated (n = 5) colonies did not differ significantly. Concentrations ranged as is shown in Table II.
As is seen in Figure 4 significant differences (P <0.05) were observed in the groups in different samplings. Significant positive correlation (r = 0.91; P <0.05) was found between coumaphos concentration in beeswax from treated and non-treated hives.
Coumaphos concentration in beeswax. A b c: values with the same superscripts differ significantly (P < 0.05). Legend: see Fig. 3.
4 Discussion
To the best of our knowledge, this is the first field study which followed coumaphos residues accumulation in three chemically different matrices in beehive following the use of coumaphos as a drug to treat varroosis. From the aspect of accumulation, honey and bee brood are closely related to beeswax since both of them are in direct contact with wax-built combs. Thus, beside contamination of all three matrices by bees carrying coumaphos on their bodies, migration of coumaphos from one matrix to another is another possible route of contamination (Kochansky et al. 2001). The rate of coumaphos migration varies depending on its solubility in each matrix. Literature data show low accumulations of coumaphos in honey. Some studies showed that coumaphos concentration in honey was not found higher than 40 μg/kg (Van Buren et al. 1992; Maver and Poklukar 2003; Valdovinos-Flores et al. 2016), although at the end of treatment with coumaphos in strips (Checkmite, Bayer, Germany) its concentration can rise up to 131 μg/kg (Karazafiris et al. 2008) or even more than 2 mg/kg (Martel et al. 2007) and decreases afterwards. In the monitoring of environmental pollution, low levels of coumaphos (2.48 ± 5.69 μg/kg) were detected in honey (Lambert et al. 2013). Similar to some previous studies, in our study, concentrations of coumaphos in honey before treatment and on the day of the removal of coumaphos strips were found below 50 μg/kg in both control and treated hives. We believe that non-detectable coumaphos levels were found due to its low water solubility (Karazafiris et al. 2008) which explains its minimal accumulation in honey. It was reported that coumaphos can intensively migrate from beeswax to honey if its concentration in beeswax reaches 1000 ppm (Kochansky et al. 2001). Therefore, the coumaphos level in honey is a result of contamination of the honey by bees (during the treatment) and the migration of coumaphos from beeswax. In our study, coumaphos concentration in beeswax did not reach 1000 ppm (1 × 106 μg/kg); thus, coumaphos migration from beeswax to honey might be excluded. Therefore, the only way to contaminate honey was bees carrying coumaphos on their bodies. As described by Valdovinos-Flores et al. (2016) during the treatment, coumaphos was found in honey of 64% tested beehives and several studies showed residue levels below 40 μg/kg (van Buren et al. 1992; Valdovinos-Flores et al. 2016). Considering these reports, we believe that contamination of honey in our study was too low to be detected with the used method. Nevertheless, our results show that coumaphos level in honey on the last day of treatment with coumaphos strips did not reach half of regulated MRL.
In contrast, coumaphos was determined in the bee brood of treated bee colonies at the end of the treatment whereas before treatment and in controls it was not found (Figure 3).
In the literature, we did not find data about levels of coumaphos residues in bee brood. Thus, to the best of our knowledge, this is the first study which shows accumulation of coumaphos in developing larvae. These results are of a great importance since the toxicity of coumaphos for developing larvae was described (Zhu et al. 2014). Unfortunately, we cannot estimate observed coumaphos levels in bee brood as high or low since there is no published data about rate of coumaphos accumulation in bee brood. Therefore, results of our study confirm coumaphos accumulation in bee brood and this must be taken into account when using coumaphos.
Coumaphos is dispersed through the colony by bees and thus comes in contact with the bee brood. In contrast to honey, larvae are relatively rich in lipids (Crane 1990); therefore, coumaphos as a fat-soluble compound can accumulate in them. Another way of contamination of larvae is, most likely, the migration of coumaphos from beeswax. In this study, coumaphos was found only in the brood from treated hives. This result suggests that larvae are contaminated through the bees carrying coumaphos on their legs and bodies during the presence of coumaphos strips in the hive. Since coumaphos was found in beeswax from all the observed hives (Figure 4) and coumaphos concentration in the brood was found below 50 μg/kg in all hives before treatment as well as in controls, we believe that coumaphos levels in beeswax did not reach a concentration at which it began to migrate from beeswax to larvae. Since larvae contain more lipids than honey (Crane 1990), it was expected that coumaphos would migrate from beeswax into larvae even if the beeswax contained coumaphos concentration lower than was described for migration into honey (Kochansky et al. 2001). In the literature, we did not find any data on the critical level of coumaphos concentration in beeswax in which coumaphos starts to migrate into developing larvae. From this point of view, determination of the critical concentration of coumaphos in beeswax might be a topic for further studies; however, this concentration is assumed to be higher than 17,000 μg/kg which is the highest determined concentration in wax from non-treated hives in this study (Table I) and lower than 1000 ppm (1 × 106 μg/kg) as determined by Kochansky et al. (2001).
Coumaphos was detected in beeswax from all examined hives in all samplings. It was also found in all wax samples that were collected before the beginning of the treatment, which is (most likely) a result of the coumaphos accumulation from previous treatments. Contamination of the beeswax potentially occurred also through wax foundations since it was reported that coumaphos accumulates in wax and is not degraded during heating and melting (Bogdanov et al. 1998; Martel et al. 2007). Therefore, similarly to previous studies (Tremolada et al. 2004; Serra-Bonvehí and Orantes-Bermejo 2010; Boi et al. 2016), the presence of coumaphos in beeswax was observed. Moreover, as is obvious from Figure 4, coumaphos concentration in beeswax increased during the experiment. The concentration of coumaphos determined in this study is somewhat higher than was found in other studies (Martel et al. 2007; Mullin et al. 2010; Boi et al. 2016); however, Bogdanov et al. (1998) and Berry et al. (2013) found higher concentrations. It was described by Berry et al. (2013) that during the treatment coumaphos concentration in beeswax reached 514,000 ppb (514,000 μg/kg). In our study, concentrations are lower; however, during the experiment, coumaphos concentration increased (Figure 4). The study by Berry et al. (2013) and our study cannot be directly compared since sampling procedures and observed beehives were not equal. However, both studies show accumulation of coumaphos in beeswax after its use for Varroa treatment. Moreover, our study shows that coumaphos concentration increased not only in the beeswax of treated hives but also of non-treated ones. The increase of coumaphos concentration in the beeswax of non-treated hives might be explained by the fact that bees from the treated hives carried coumaphos on their bodies and visited other nearby colonies and thus contaminated the beeswax. Since it was estimated that more than 15% of bees in the colony might be drifters (Forfert et al. 2015), the transmission of coumaphos from treated to non-treated colonies seems very likely. Moreover, as illustrated by Wu et al. (2011), pesticide residues could migrate through or across comb wax, which might be an additional reason for increased coumaphos levels in non-treated hives. Curiously, coumaphos was not detected in the bee brood from non-treated hives, although the wax from these hives was contaminated. As discussed above, coumaphos concentration in beeswax was probably too low to migrate from the beeswax and contamination of larvae by contaminated bees was also negligible.
Considering the results of studies by Bogdanov et al. (1998), Martel et al. (2007), Mullin et al. (2010), and Boi et al. (2016) and the results of this study, it might be assumed that the concentration of coumaphos in beeswax is a result of several factors: use of contaminated wax foundations, contamination of the beeswax from previous treatments, distance between coumaphos strip location and the location of wax sample collection, frequency of the treatment with coumaphos, coumaphos use in bee colonies in the area, and coumaphos use as a veterinary drug in other species with possible contacts with bees. Therefore, coumaphos concentration in beeswax is an important factor that represents a dormant threat for contamination of larvae or even honey. To avoid coumaphos contamination of bee products and developing larvae, rotation of acaricides in bee treatment is strongly recommended. Coumaphos should be used only in strongly affected bee colonies. Additionally, control of the coumaphos content in beeswax and use of low-contaminated wax could reduce the risk of harmful coumaphos action on bee colonies and on contamination of bee products.
As concluded, results of this 2-year field study show that following treatment against varroosis coumaphos is accumulated in some matrices in beehive. Thus, coumaphos does not exhibit a risk for the contamination of honey, until its beeswax concentration reaches a critical level at which it starts to migrate from comb to honey (Kochansky et al. 2001). During the treatment, coumaphos accumulates in the bee brood. In beeswax, the accumulation of coumaphos was determined in hives in which it was used and in hives nearby in which it was not used. Additionally, after coumaphos use for the routine treatment of varroosis in two consecutive years, an increase of its concentration in beeswax was observed.
References
Berry, J.A., Hood, W.M., Pietravalle, S., Delaplane, K.S. (2013) Field-level subletal effects of approved bee hive chemicals on honey bees (Apis melifera L). PLoS One, DOI: 10.1371/journal.pone.0076536
Bogdanov, S., Kilchenmann, V., Imdorf, A. (1998) Acaricide residues in some bee. J. Apic. Res. 37, 57–67.
Boi, M., Serra, G., Colombo, R., Lodesani, M., Massi, S., Costa, C. (2016) A 10 year survey of acaricide residues in beeswax analysed in Italy. Pest Manag. Sci. 72, 1366–1372
Crane, E. (1990) Bees and beekeeping; science, practice and world resources. Heinemann Newnes, Oxford.
European Commission (2010) Commission regulation No. 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin [online] http://ec.europa.eu/health/files/eudralex/vol-5/reg_2010_37/reg_2010_37_en.pdf (accessed on 11 August 16)
Forfert, N., Natsopoulou, M.E., Frey, E., Rosenkranz, P., Paxton, R.J., Moritz, R.F.A. (2015) Parasites and pathogens of the oneybee (Apis mellifera) and their influence on inter-colonial transmission. PLoS One, DOI: 10.1371/journal.pone.0140337
Karazafiris, E., Tananaki, C., Menkissoglu-Spiroudi, U., Thrasyvoulou, A. (2008) Residue distribution of the accaricide coumaphos in honey following application of a new slow-release formulation. Pest Manag. Sci. 64, 165–171
Kochansky, J., Wilzer, K., Feldlaufer, M. (2001) Comparison of the transfer of coumaphos from beeswax into syrup and honey. Apidologie 32, 589–601
Lambert, O., Piroux, M., Puyo, S., Thorin, C., L'hostis, M., Wiest, L., Buleté, A., Delbac, F., Pouliquen, H. (2013) Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of Western France. PLoS One, DOI: 10.1371/journal.pone.0067007
Martel, A.C., Zeggane, S. (2002) Determination of acaricides in honey by high-performance liquid chromatography with photodiode array detection. J. Chromatogr. A 954, 173–80
Martel, A.C., Zeggane, S., Aurieres, C., Drajnudel, P., Faucon, J.P., Aubert, M. (2007) Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar or Asuntol 50. Apidologie 38, 534–544
Maver, L., Poklukar, J. (2003) Coumaphos and amitraz residues in Slovenian honey. Apiacta 38, 54–57
McDonnell, C.M., Alaux, C., Parrinello, H., Desvignes, J.-P., Crauser, D., Durbesson, E., Beslay, D., Le Conte, Y. (2013) Ecto- and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC Ecol. 13(1), 25
Millar, N.S., Denholm, I. (2007) Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invertebr. Neurosci. 7, 53–66
Mullin, C.A., Frazier, M., Frazier, J.L., Ashcraft, S., Simonds, R., van Engelsdorp, D., Pettis, J.S. (2010) High level of miticides and agrochemicals in North American apiaries: implications for honey bee health. Plos One, DOI: 10.1371/journal.pone.0009754
Ravoet, J., Reybroeck, W., de Graaf, D.C. (2015) Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 94, 543–548
Serra-Bonvehí, J., Orantes-Bermejo, J. (2010) Acaricides and their residues in Spanish commercial beeswax. Pest Manag. Sci. 66, 1230–1235
Tabart, J., Colin, M.E., Carayon, J.L., Tene, N., Payre, B., Vetillard, A. (2013) Artificial feeding of Varroa destructor through a chitosan membrane: a tool for studying the host-microparasite relationship. Exp. Appl. Acarol. 61, 107–118
Tremolada, P., Bernardinelli, I., Colombo, M., Spreafico, M., Vighi, M. (2004). Coumaphos distribution in the hive ecosystem: case study for modeling applications. Ecotoxicol. 13, 589–601
Valdovinos-Flores, C., Gaspar-Ramírez, O., Heras-Ramírez, M.E., Lara-Álvarez, C., Dorantes-Ugalde, J.A., Saldaña-Loza, L.M. (2016). Boron and coumaphos residues in hive materials following treatments for the control of Aethina tumida Murray. PLoS One, DOI: 10.1371/journal.pone.0153551
Van Buren, N.W.M., Mariën, A.G.H., Velthuis, H.H.W., Oudejans, R.C.H.M. (1992) Residues in beeswax and honey of Perezin, an acaricide to combat the mite Varroa jacobsoni Oudemans (Acari: Mesostigmata). Environ. Entomol. 21, 860–865
Wu, J.Y., Anelli, C.M., Sheppard, W.S. (2011) Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PloS One, DOI: 10.1371/journal.pone.0014720
Xu, J.Z., Miao, J.J., Lin, H., Ding, T., Zhao, Z.Y., Wu, B., Shen, C.Y., Jiang, Y. (2009) Determination of amitraz and 2,4-dimethylaniline residues in honey by using LC with UV detection and MS/MS. J. Sep. Sci. 32; 4020–4024
Zhu, W., Schmehl, D.R., Mullin, C.A., Frazier, J.L. (2014) Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS One, DOI: 10.1371/journal.pone.0077547
Acknowledgements
The work was financially supported by the Slovenian Research Agency and Ministry of Agriculture, Forestry and Food of the Republic of Slovenia (Grants No. V4-1105 and P4-0053). We are grateful to Terry T. Jackson for English editing.
Authors’ contribution
BPB, TS and VJ: design of the study. AF: validation of analytical methods. BPB, TS, LM, VJ and DŠ: preparation of the manuscript. SK and TS: organization of the laboratory and field work. VJ: organization of the sample collection in the field. MPO and MŠ: sample collection in the field.
BPB, KB, TS, LM, MPO, MŠ, VJ, AF, DŠ, and SK: interpretation of the results. TS: statistics.
BPB, KB, and LM: chemical analyses of coumaphos in honey and bee brood. DŠ: analyses of coumaphos in beeswax.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Résidus de coumaphos dans le miel, le couvain d’abeille et la cire après traitement contre le varroa
Acaricide/ produit de la ruche/ abeille/ contamination/ organophosphate
Coumaphos-Rückstande in Honig, Bienenbrut und Bienenwachs nach Varroa-Behandlung
Akarizid / Bienenprodukte / Honigbiene / Kontamination / Organophosphate
Rights and permissions
About this article
Cite this article
Premrov Bajuk, B., Babnik, K., Snoj, T. et al. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 48, 588–598 (2017). https://doi.org/10.1007/s13592-017-0501-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-017-0501-y