Abstract
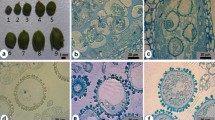
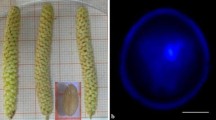
Composition of nutrient media, flower bud size, sucrose concentration, heat shock stress, and ethylene inhibitor could have marked effects on microspore embryogenesis. No microspore-derived embryos (MDE) were formed when the microspores were isolated from radish (Raphanus sativus L.) flower buds of 1.0–2.5 mm in size, whereas MDE were formed with microspores isolated from 2.5–4.5 and 4.5–6.5 mm flower buds. The microspores isolated from 2.5–4.5 mm flower buds showed high embryo yields. MDE formation was highest when 150 g·L−1 sucrose was added to the half strength Nitsch & Nitsch (NLN) liquid medium, but at sucrose concentrations less than 100 g·L−1 there was no MDE formation. Microspores cultured on half strength NLN liquid medium containing 0.05 mg·L−1 silver nitrate (AgNO3) produced the most MDE, showing more than two-fold increase in yield compared to those cultured on medium without AgNO3. A heat shock pretreatment of microspores at 32°C for 24 h gave high-frequency production of MDE when compare to higher or lower temperatures; no MDE were formed at 42.5°C. The highest yield of MDE was observed when microspores were derived from 2.5–4.5 mm flower buds cultured on half strength NLN medium containing 150 g·L −1 sucrose, 0.05 mg·L−1 AgNO3, and precultured with heat shock pretreatment of microspores at 32°C for 24 h, followed by incubation 25°C for 30 days. A polyploidy test indicated that 19.7% of the microspore-derived plants were doubled haploid, other plants were haploid, and chimeras were haploid and diploid.
Similar content being viewed by others
Literature Cited
Barro, F. and A. Martin. 1999. Response of different genotypes of Brassica carinata to microspore culture. Plant Breed. 118:79–81.
Barro, F., J. Fernandez-Escobar, M. De la Vega, and A. Martin. 2003. Modification of glucosinolate and erucic acid contents in doubled haploid lines of Brassica carinata by UV treatment of isolated microspores. Euphytica 129:1–6.
Beyer, E.M. 1976. A potent inhibitor of ethylene action in plants. Plant Physiol. 58:268–271.
Biddington, N.L. 1992. The influence of ethylene in plant tissue culture. Plant Growth Regulat. 11:173–187.
Binarova, P., G. Hause, V. Cenklova, J.H.G. Cordewener, and M.M.L. Campagne. 1997. A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex. Plant Reprod. 10:200–208.
Cao, M.Q., Y. Li, F. Liu, and C. Dore. 1994. Embryogenesis and plant regeneration of pakchoi (Brassica rapa L. ssp. chinensis) via in vitro isolated microspore culture. Plant Cell Rpt. 13:447–450.
Chen, W.H., C.Y. Tang, and Y.L. Kao. 2009. Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tissue Organ Cult. 98:229–238.
Curtis, I.S. 2009. Radish, p. 381–389. In: E.C.V. Pua and M.R. Davey (eds.). Biotechnology in agriculture and forestry. Springer, Dordrecht.
Evans, J.M. and N.P. Vatty. 1994. Ethylene precursors and antagonists increase embryogenesis of Hordeum vulgare L. Anther Cult. Plant Cell Rpt. 13:676–678.
Ferrie, A.M.R. 2009. Current status of doubled haploids in medicinal plants, p. 209–217. In: A. Touraev, B.P. Forster, and S.M. Jain (eds.). Advances in haploid production in higher plants. Springer, Dordrecht.
Friedt, W. and M.K. Zarhloul. 2005. Haploids in the improvement of Crucifers, p. 191–213. In: C.E. Plamer, W.A. Keller, K.J. Kasha (eds.). Biotechnology in agriculture and forestry. Springer, Dordrecht.
Gamborg, O.L. and T.A.G. Larue. 1971. Ethylene production by plant cell cultures. Plant Physiol. 48:399–401.
Gamborg, O.L., R.A. Millar, and K. Ojima. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158.
Gik-Humanes, J. and F. Barro. 2009. Production of doubled haploids in Brassica, p. 65–73. In: A. Touraev, B.P. Forster, and S.M. Jain (eds.). Advances in haploid production in higher plants. Springer, Dordrecht.
Gu, H.H., W.J. Zhou, and P. Hagberg. 2003. High frequency spontaneous production of doubled haploid plants in microspore cultures of Brassica rapa ssp. chinensis. Euphytica 134:239–245.
Kott, L.S., L. Polsoni, B. Ellis, and W.D. Beversdorf. 1988. Autotoxicity in isolated microspore cultures of Brassica napus. Can. J. Bot. 66:1665–1670.
Lichter, R. 1982. Induction of haploid plants from isolated pollen of Brassica napus. Z. Pflanzenphysiol. 105:427–434.
Lionneton, E., W. Beuret, C.O. Delaitre, S. Chatt, and M. Rancillac. 2001. Improved microspore culture and doubled-haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rpt. 20:126–130.
Mensuari, S.A., M. Panizza, and E. Tognoni. 1992. Quantification of ethylene losses in different container seal systems and comparison of biotic and abiotic contributions to ethylene accumulation in cultured tissues. Physiol. Plant. 84:472–476.
Mishiba K, T. Ando, M. Mii, H. Watanabe, H. Kokubun, G. Hashimoto, and E. Marchesi. 2000. Nuclear DNA content as an index character discriminating taxa in the genus Petunia sensu Jussieu (Solanaceae). Ann. Bot. 85:665–673.
Olmedilla, A. 2010. Microspore embryogenesis, p. 27–44. In: E.C. Pua and M.R. Davey (eds.). Plant developmental biology-biotechnological perspectives. Springer, Dordrecht.
Pechan, P.M. and P. Smykal. 2001. Androgenesis: Affecting the fate of the male gametophyte. Physiol. Plant. 111:1–8.
Phippen, C. 1990. Genotype plant bud size and media factors affecting anther culture of cauliflowers (Brassica oleracea var. botrytis). Theor. Appl. Genet. 79:33–38.
Prem, D., K. Gupta, and A. Agnihotri. 2005. Effect of various exogenous and endogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea (L.) Czern and Coss). In Vitro Cell. Dev. Biol. Plant 41:266–273.
Siebel, J. and K.P. Pauls. 1989. A comparison of anther and microspore culture as a breeding tool in Brassica napus. Theor. Appl. Genet. 78:473–479.
Telmer, C.A., D.H. Simmonds, and W. Newcomb. 1992. Determination of development stage to obtain high frequencies of embryogenic microspores in Brassica napus. Physiol. Plant. 84:417–424.
Tiainen, T. 1992. The role of ethylene and reducing agents on anther culture response of tetraploid potato (Solanum tuberosum L.). Plant Cell Rpt. 10:604–607.
Wang, M., M.W. Farnham, and J.S.P. Nannes. 1999. Ploidy of broccoli regenerated from microspore culture versus anther culture. Plant Breed. 118:249–252.
Weber, S., F. Unker, and W. Friedt. 2005. Improved doubled haploid production protocol for Brassica napus using microspore colchicines treatment in vitro and ploidy determination by flow cytometry. Plant Breed. 124:511–513.
Wedzony, M., B.P. Forster, I. Zur, E. Golemiec, M. Szechynska-Hebda, E. Dubas, and G. Gotebiowska. 2009. Progress in doubled haploid technology in higher plants, p. 1–33. In: A. Touraev, B.P. Forster, and S.M. Jain (eds.). Advances in haploid production in higher plants. Springer, Dordrecht.
Wong, R.S.C., S.Y. Zee, and E.B. Swanson. 1996. Isolated microspore culture of Chinese flowering cabbage (Brassica campestris ssp. parachinensis). Plant Cell Rpt. 15:396–400.
Wu, L.M., Y.M. Wei, and Y.L. Zheng. 2006. Effects of silver nitrate on the tissue culture of immature wheat embryos. Russian Plant Physiol. 53:592–596.
Yang, S.F. and N.E. Hoffman. 1984. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35:155–189.
Zaki, A.M. and H.G. Dickinson. 1990. Structural changes during the first divisions of embryos resulting from anther and free microspore culture in B. napus. Protoplasma 156:149–162.
Zhou, W.W.J., G.G.X. Tang, and P.P. Hagberg. 2002. Efficient production of doubled haploid plants by immediate colchicines treatment of isolated microspores in winter Brassica napus. Plant Growth Regulat. 37:185–192.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chun, C., Park, H. & Na, H. Microspore-derived embryo formation in radish (Raphanus sativus L.) according to nutritional and environmental conditions. Hortic. Environ. Biotechnol. 52, 530–535 (2011). https://doi.org/10.1007/s13580-011-0080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-011-0080-1