Abstract
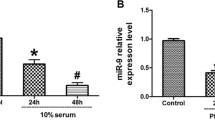
To investigate the effects of miR-98 on TGF-β1-induced cardiac fibrosis in human cardiac fibroblasts (HCFs), and to establish the mechanism underlying these effects, HCFs were transfected with miR-98 inhibitor or mimic, and then treated with or without TGF-β1. The level of miR-98 was determined by qRT-PCR in TGF-β1-induced HCFs. Cell differentiation and collagen accumulation of HCFs were detected by qRT-PCR and Western blot assays, respectively. The mRNA and protein expressions of TGFBR1 were determined by qRT-PCR and Western blotting. In this study, the outcomes showed that TGF-β1 could dramatically decrease the level of miR-98 in a time- and concentration-dependent manner. Upregulation of miR-98 dramatically improved TGF-β1-induced increases in cell differentiation and collagen accumulation of HCFs. Moreover, bioinformatics analysis predicted that the TGFBR1 was a potential target gene of miR-98. Luciferase reporter assay demonstrated that miR-98 could directly target TGFBR1. Inhibition of TGFBR1 had the similar effect as miR-98 overexpression. Downregulation of TGFBR1 in HCFs transfected with miR-98 inhibitor partially reversed the protective effect of miR-98 overexpression on TGF-β1-induced cardiac fibrosis in HCFs. Upregulation of miR-98 ameliorates TGF-β1-induced differentiation and collagen accumulation of HCFs by downregulation of TGFBR1. These results provide further evidence for protective effect of miR-98 overexpression on TGF-β1-induced cardiac fibrosis.






Similar content being viewed by others
References
Zhou Y, Deng L, Zhao D, et al. MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016;20(3):495–505.
Zeigler AC, Richardson WJ, Holmes JW, et al. Computational modeling of cardiac fibroblasts and fibrosis. J Mol Cell Cardiol. 2015;93:73–83.
Tao H, Shi KH, Yang JJ, et al. Epigenetic regulation of cardiac fibrosis. Cell Signal. 2013;25(9):1932–8.
Tijsen AJ, van der Made I, van den Hoogenhof MM, et al. The microRNA-15 family inhibits the TGFbeta-pathway in the heart. Cardiovasc Res. 2014;104(1):61–71.
Jiang X, Tsitsiou E, Herrick SE, et al. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277(9):2015–21.
Dai Y, Khaidakov M, Wang X, et al. MicroRNAs involved in the regulation of postischemic cardiac fibrosis. Hypertension. 2013;61(4):751–6.
van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015;93:133–42.
Zhao X, Wang K, Liao Y, et al. MicroRNA-101a inhibits cardiac fibrosis induced by hypoxia via targeting TGFbetaRI on cardiac fibroblasts. Cell Physiol Biochem. 2015;35(1):213–26.
Bei Y, Song Y, Wang F, et al. miR-382 targeting PTEN–Akt axis promotes liver regeneration. Oncotarget. 2016;7(2):1584–97.
Xu T, Zhou Q, Che L, et al. Circulating miR-21, miR-378, and miR-940 increase in response to an acute exhaustive exercise in chronic heart failure patients. Oncotarget. 2016;7(11):12414–25.
Liu X, Xiao J, Zhu H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95.
Liang D, Xu X, Deng F, et al. miRNA-940 reduction contributes to human tetralogy of fallot development. J Cell Mol Med. 2014;18(9):1830–9.
Xiao J, Liang D, Zhang H, et al. MicroRNA-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. J Mol Cell Cardiol. 2012;53(6):751–9.
Mai L, Xiao L, Huang Y, et al. Novel microRNAs involved in regulation of cardiac fibrosis. Int J Cardiol. 2015;192:14–5.
Gupta SK, Itagaki R, Zheng X, et al. miR-21 promotes fibrosis in an acute cardiac allograft transplantation model. Cardiovasc Res. 2016;110(2):215–26.
Huang Y, Qi Y, Du JQ, et al. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets. 2014;18(12):1355–65.
Nagpal V, Rai R, Place AT, et al. MiR-125b is critical for fibroblast-to-myofibroblast transition and cardiac fibrosis. Circulation. 2016;133(3):291–301.
Wang X, Wang HX, Li YL, et al. MicroRNA Let-7i negatively regulates cardiac inflammation and fibrosis. Hypertension. 2015;66(4):776–85.
Wang L, Ma L, Fan H, et al. MicroRNA-9 regulates cardiac fibrosis by targeting PDGFR-β in rats. J Physiol Biochem. 2016;72(2):213–23.
Tao H, Chen ZW, Yang JJ, et al. MicroRNA-29a suppresses cardiac fibroblasts proliferation via targeting VEGF-A/MAPK signal pathway. Int J Biol Macromol. 2016;88:414–23.
Sang HQ, Jiang ZM, Zhao QP, et al. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed Pharmacother. 2015;71:185–9.
Zhu W, Yang L, Shan H, et al. MicroRNA expression analysis: clinical advantage of propranolol reveals key microRNAs in myocardial infarction. PLoS One. 2011;6:e14736.
Tao H, Yang JJ, Hu W, et al. Noncoding RNA as regulators of cardiac fibrosis: current insight and the road ahead. Pflugers Arch. 2016;468(6):1103–11.
Tao H, Yang JJ, Shi KH, et al. Wnt signaling pathway in cardiac fibrosis: new insights and directions. Metabolism. 2016;65(2):30–40.
Sun M, Yu H, Zhang Y, et al. MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep. 2015;5:18351.
Pellman J, Lyon RC, Sheikh F. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J Mol Cell Cardiol. 2010;48(3):461–7.
Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89(2):265–72.
Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–75.
Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;987(9):9800–7.
Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–78.
van den Borne SW, Diez J, Blankesteijn WM, et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7(1):30–7.
Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 2009;6(6):848–56.
Leask A. Potential therapeutic targets for cardiac fibrosis TGFβ, angiotensin, endothelin, CCN2 and PDGF, partners in fibroblast activation. Circ Res. 2010;106(11):1675–80.
Pelouch V, Dixon IM, Golfman L, et al. Role of extracellular matrix proteins in heart function. Mol Cell Biochem. 1993; 129(2): 101–20.
Zhong C, Wang K, Liu Y, et al. miR-198b controls cardiac fibroblast proliferation and migration. J Cell Mol Med. 2016;20(6):11981–7.
Zhou Y, Deng L, Zhao D, et al. MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016;20(3):4985–5505.
Nagpal V, Rai R, Place AT, et al. MiR-125b is critical for fibroblast-to-myofibroblast transition and cardiac fibrosis. Circulation. 2016;133(3):2981–3301.
Zhang Y, Huang XR, Wei LH, et al. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. 2014;22(5):974–85.
Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genetic Metabol. 2000;71(1–2):418–35.
Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106(1):130–5.
Lim H, Zhu YZ. Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci. 2006;63(22):2584–96.
Chen J, Mehta JL. Angiotensin II-mediated oxidative stress and procollagen-I expression in cardiac fibroblasts: blockade by pravastatin and pioglitazone. Am J Physiol Heart Circ Physiol. 2006;291(4):H1738–45.
Liu X, Sun SQ, Hassid A, et al. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70(6):1992–2003.
Driesen RB, Nagaraju CK, Abi-Char J, et al. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. 2014;101(3):411–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheng, R., Dang, R., Zhou, Y. et al. MicroRNA-98 inhibits TGF-β1-induced differentiation and collagen production of cardiac fibroblasts by targeting TGFBR1. Human Cell 30, 192–200 (2017). https://doi.org/10.1007/s13577-017-0163-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-017-0163-0