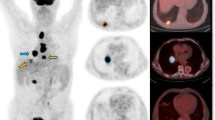
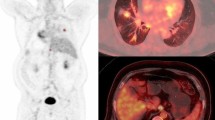
Abstract
Objective
This study aimed to (1) compare the agreement of two evaluation methods of metabolic response in patients with non-small cell lung cancer (NSCLC) and determine their prognostic value and (2) explore an optimal cutoff of metabolic reduction to distinguish a more favorable subset of responders.
Methods
This is a secondary analysis of prospective studies. Enrolled patients underwent 18F-PET/CT within 2 weeks before, during, and months after radiotherapy (post-RT). Metabolic response was assessed using both Peter MacCallum (PM) method of qualitative visual assessment and University of Michigan (UM) method of semiquantitative measurement. The agreement between two methods determined response, and their prediction of outcome was analyzed.
Results
Forty-four patients with median follow-up of 25.2 months were analyzed. A moderate agreement was observed between PM- and UM-based response assessment (Kappa coefficient = 0.434), unveiling a significant difference in CMR rate (p = 0.001). Categorical responses derived from either method were significantly predictive of overall survival (OS) and progression-free survival (PFS) (p < 0.0001). Numerical percentage decrease of FDG uptake also showed significant correlations with survival, presenting a hazard ratio of 0.97 for both OS and PFS. A 75 % of SUV decrease was found to be the optimal cutoff to predict OS and 2-year progression.
Conclusions
There was a modest discrepancy in metabolic response rates between PM and UM criteria, though both could offer predictive classification for survival. The percentage decrease provides an ordinal value that correlates with prolonged survival, recommending 75 % as the optimal threshold at identifying better responders.
Similar content being viewed by others
Introduction
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT)-based metabolic response has been reported to be a prognostic indicator for patients with non-small cell lung cancer (NSCLC) by a large number of studies [1–22]. However, there is a wide variation in the measurement of tumor FDG uptake and the stratification into response categories, resulting in the fact that multiple criteria for metabolic response evaluation are proposed and applied diversely among clinical centers. Currently, methods for evaluating metabolic response on FDG-PET include qualitative visual assessment such as Peter MacCallum (PM) criteria [5], semiquantitative assessment using reduction of metabolic activity (e.g., SUV) such as EORTC [23], University of Michigan (UM) [10], and PERCIST [24] methods, and quantitative evaluation using kinetic modeling [25].
Quantitative method of kinetic modeling or Patlak plot is used infrequently in clinical practice due to the complexity, including the needs for arterial blood sampling or dynamic imaging of a blood-pool structure to acquire a precise input function [25]. The best method of metabolic response evaluation is currently unknown, both for predicting outcome and being easily applicable into routine clinic practice. All of the aforementioned response criteria are currently being used in clinical practice, and there was no direct cross-comparison between the various methods yet.
With regard to semiquantitative assessment, many studies have reported that over 70 % of NSCLC patients underwent at least 30 % of posttreatment decrease in metabolic activity, namely the partial metabolic response (PMR) by semiquantitative evaluation methods [9, 10, 13, 17, 20]. This indicates that the current cutoff values of 25 or 30 % of metabolic decrease for PMR are probably too modest or insufficient to identify medically relevant response. Given this consideration, it would be advantageous to explore another reliable cutoff value that might allow distinguishing a subgroup of PMR patients with more favorable outcome.
This study aimed to (1) compare response rates of PM (a qualitative method) and UM method (a semiquantitative) on FDG PET/CT and examine their value for predicting long-term outcome and (2) perform a post hoc analysis to explore an optimal cutoff for the selection of better responders in NSCLC treated with definitive radiotherapy.
Methods
Study population
The patients in this study represented a cohort from a series of functional imaging-related prospective studies between 2003 and 2010 at the University of Michigan (UM). These studies were approved by the institutional review board (IRB) of the University of Michigan, and written informed consent was obtained from each patient. Unresectable or inoperable stage I–III NSCLC patients receiving definitive radiation therapy (RT) underwent FDG PET/CT scans within 2 weeks prior to RT beginning (pre-RT PET/CT) and at the fourth week during RT as per protocol. After treatment, patients were followed up with CT with or without PET/CT per physician’s discretion, and those with PET/CT scans within 6 months after RT (post-RT PET/CT) were eligible for the present analysis. Exclusion criteria included prior thoracic RT history, small cell carcinoma histology, and comorbidity of diabetes mellitus with uncontrolled blood glucose level >200 mg/dL. All patients had a definitive course of radiotherapy under standard practice or per protocol. As we are focusing on posttreatment response assessment, we did not restrict the RT regimens.
PET/CT image acquisition
FDG PET/CT scans were performed at two institutions: University of Michigan Hospital (UMH) and Veterans Administration Health Center/Veterans Affairs Medical Center, Ann Arbor (VA-AA) between 2003 and 2010. The PET protocols used at both institutions were standardized throughout this time period. Details of the PET/CT scan protocols have been previously described [10]. At the UMH between 2003 and 2006, the PET/CT imaging was performed on a Siemens Biograph Classic (Siemens Medical Solutions, Hoffman Estates, IL, USA) and between 2006 and 2010 on a Siemens Biograph T6. All PET/CT studies at the VA Ann Arbor Medical Center were performed on a Siemens Biograph T6.
Image interpretation and response evaluation
Interpretation of PET/CT imagings was performed by nuclear medicine physicians blinded to clinical information using consensus criteria. Mean SUV (SUVmean) within a circular region of interest (ROI) of about 1.0-cm diameter placed in the aorta arch (AA) was used to represent the metabolic activity of mediastinal blood pool (MBP) for purpose of normalization. Tumor FDG uptake expressed as normalized maximum SUV (SUVmax) to AA (NSUV-A) was calculated using the following formula [10, 26, 27]:
Each patient’s set of PET/CT studies was evaluated twice using PM and UM methods, respectively [5, 10]. The numerical percentage decline of NSUV-A was also analyzed as a continuum.
Statistical analysis
Kappa coefficient was used to estimate the agreement between PM and UM evaluation systems. Overall response to treatment (ORTT) was determined based on primary tumor (T), regional lymph nodes (N), and distant status (M). Infield response to treatment (IFRTT) was defined as the response to treatment regarding only lesions within the radiation field. For instance, the case in which new lesions appear and progress despite apparent remission of the local disease would be classified as PMD of ORTT whereas CMR of IFRTT. We explored 2-year progression as the endpoint to separate study patients into two groups (early progression vs. late progression). Receiver operating characteristic (ROC) analysis was used to identify the optimal cutoff in percentage decline of NSUV-A to discriminate the two groups. Kaplan–Meier survival analysis and log-rank test were performed to compare survival between different groups. Using cutoffs varying from 25 to 90 % reduction of NSUV-A at 5 % increments, the discriminative values of various cutoffs were analyzed for both overall survival (OS) and progression-free survival (PFS) by Kaplan–Meier method. Cox proportional hazard regression was rendered to investigate the prognostic significance of various response systems. A p ≤ 0.05 was considered statistically significant, and all tests were two-tailed.
Results
General characteristics
A total of 118 patients were enrolled in the prospective studies, and eventually, 44 patients with both pre- and post-RT scans were eligible for this analysis. Table 1 shows the demographics and tumor characteristics for the study population. There were 6 patients with adenocarcinoma, 10 with squamous cell carcinoma, 1 with large cell carcinoma, and 23 with NSCLC but unknown subtype, primarily due to the limited specimen obtained from fine needle aspiration-based biopsy. In addition, four patients had clinically diagnosed NSCLC based on the consensus of tumor board but without pathological verification because of unfitness for biopsy. The median RT dose was 70 Gy. The interval between RT end and post-RT PET/CT scan ranged from 45 to 176 days, with a median of 93 days.
Comparison of categorical metabolic response assessment by PM and UM methods
According to PM methods using qualitative visual assessment, the rates of CMR, PMR, SMD, and PMD were 38.6, 52.3, 2.3, and 6.8 %, respectively. For UM method, they were 13.6, 72.7, 9.1, and 4.5 %, respectively. A significant difference in CMR rate was observed between the UM and PM methods (p = 0.001), while no significant differences were seen for PMR, SMD, and PMD. The distribution of ORTT between PM and UM criteria is shown in Table 2, with an agreement rate of 68.1 % (30/44) and a Kappa coefficient of 0.434. Figure 1 graphs the actual change in NSUV-A with the corresponding response categories of ORTT and IFRTT determined by PM criteria, with the discrepancy in-between that resulted from two cases with infield CMR (58.4 and 87.1 % reduction in NSUV-A, respectively) but with out-of-field failure.
Categorical response and survival
The median follow-up time for the study population was 25.2 months. Figure 2a, b depicts that both PM and UM methods determined response classification were predictive of OS (p < 0.0001). Patients with CMR achieved significantly better outcome than the non-CMR population. The median survival (MS) was 48.1 and 18.3 months for PM criteria-based CMR and non-CMR patients (p = 0.016). By the end of the last follow-up, the MS was not reached for UM criteria-determined CMR patients and was 22.0 months for non-CMR patients (p = 0.086). Similar findings were observed with respect to PFS results. Both PM and UM method-based response classifications were predictive of PFS (p < 0.0001) (Fig. 2c, d). The CMR group compared to the non-CMR group had median PFS of 33.7 months vs. 8.4 months (p = 0.005) for PM criteria and 25.5 vs. 12.4 months, respectively (p = 0.231), for UM criteria.
Combining PM and UM criteria, we divided the patients into three groups: (1) those achieving CMR under both criteria (visual CMR and NSUV-A ≤ 1), (2) those identified as CMR using PM system but non-CMR using UM criteria (visual CMR but NSUV-A > 1), and (3) the remaining patients as CMR in neither group (neither visual CMR nor NSUV-A ≤ 1) for further survival analysis. A significant difference among the three groups was observed, with regard to both OS (p = 0.044, Fig. 2e) and PFS (p = 0.019, Fig. 2f), which was mainly resulted from the inferior outcome of non-CMR classification (neither visual CMR nor NSUV-A ≤ 1).
Optimal cutoff for prediction of progression and survival
Cox regression analysis showed a positive correlation between the numerical percentage decrease of NSUV-A and survival, resulting in the hazard ratio (HR) of 0.971 (95%CI, 0.955–0.988) and 0.971 (95%CI, 0.957, 0.986) from 1 % reduction of NSUV-A for OS and PFS, respectively. ROC analysis identified 75 % decrease in NSUV-A as the optimal cutoff, translating into a sensitivity of 80 % and specificity of 69 % for the 2-year progression (Fig. 3a). The discriminative values of cutoffs varying from 25 to 90 % reduction of NSUV-A at 5 % increments were analyzed for both OS and PFS. Besides the 25 % decrease proposed by EORTC criteria, a threshold of 75 and 60 % reduction was found by OS (p = 0.002) and PFS (p < 0.0001) analyses, respectively, to introduce the most discriminative significance (Fig. 3b, c). Using 25 and 75 % reduction as the cutoffs to stratify patients into three subgroups, we saw significantly different OS (Fig. 4a, 70.7 vs. 20.5 vs. 7.6 months, p < 0.0001) and PFS (Fig. 4b, 25.5 vs. 8.4 vs. 4.6, p < 0.0001) across groups.
Discussion
This study demonstrated a remarkable discrepancy in CMR rates between PM (qualitative visual assessment) and UM methods (semiquantitative assessment). Both criteria can distinguish patients with different outcomes providing important prognostic information, although the predictability may be considered somewhat coarse and suboptimal. As a continuum, the actual percentage change of metabolic activity was also shown to be predictive of survival, and a 75 % decline was found to be the optimal threshold to predict 2-year progression and the overall survival in this NSCLC patient cohort treated with definitive RT.
Metabolic response has been broadly applied in clinical studies and increasingly used in clinical practice as well. However, there is little literature investigating the agreement across various response evaluation methods and comparing their predictive value for outcome. To our knowledge, there were two published studies currently making the direct comparison of the concordance and the prognostic significance between EORTC and PERCIST criteria [28, 29]. Skougaard and colleagues found that response evaluation with EORTC criteria and PERCIST gave similar responses and OS outcomes with good agreement on best overall metabolic response in metastatic colorectal cancer treated with irinotecan and cetuximab [28]. Another study enrolling 29 patients with small cell lung cancer demonstrated a perfect concordance between EORTC and PERCIST criteria (kappa coefficient = 1) [29].
Different from the abovementioned studies, our study made a comparison between the qualitative and semiquantitative methods. Our results revealed a moderate agreement between two methods whereas significantly higher CMR rate under PM method-based visual assessment. This finding indicated that the application of various response criteria could cause different conclusion about the efficacy of the treatment, which would lead to confusion in interpretation of trial results or clinical reports and may affect the clinical decision making for the further treatment such as the maintenance therapy. One should be cautious when comparing the metabolic response results across trials without identical evaluation methods.
Our results of 44 patients confirmed findings from previous studies that metabolic response (either UM or PM methods) is predictive of outcome in NSCLC [1–24]. Posttreatment CMR clearly is associated with the longest survival irrespective of utilized methods. However, the survival differences in terms of both OS and PFS between UM criteria defined that CMR and non-CMR groups in our study did not reach statistical significance. Further stratification dividing PM criteria determined CMR patients into two subgroups (visual CMR and NSUV-A ≤ 1 vs. visual CMR but NSUV-A > 1) that failed to find further distinction in the context of OS or PFS, suggesting that the semiquantitative assessment of CMR may be too strict to miss good responders with only mild FDG uptake undiscerned by visual evaluation.
Interestingly, our findings demonstrated that the actual percentage reduction in NSUV-A was positively correlated with OS and PFS in a continuous variable model, which was consistent with the findings of ACRIN 6668/RTOG 0235 Trial [30]. This suggests that every single percent of metabolic activity reduction may confer a certain survival advantage and the relationship between tumor metabolic change and prognosis may be continuous rather than having a fixed cutoff point. Nonetheless, the categorical classification of metabolic response is a practical approach for evaluating treatment efficacy and guiding clinical decisions in daily care.
In this study, we validated that less than 25 or 30 % decrease in metabolic activity applied by EORTC and UM criteria would have allowed for the discrimination of non-responders (SMD) from PMR patients. However, many studies have reported that over 70 % of NSCLC patients underwent at least 30 % of metabolic decrease [9, 10, 13, 17, 20]. Therefore, it is rational to raise the hypothesis that there may be a subset of patients with more favorable outcome in the UM or EORTC criteria determined PMR population. Several studies have attempted to modify cutoffs of posttreatment metabolic change to optimize the categorical response evaluation. Shiraishi and colleagues found that postneoadjuvant chemoradiotherapy of 40 % decrease in SUV could predict pathological response but could not successfully predict long-term overall survival [20]. Eschmann and colleagues proposed postneoadjuvant chemoradiotherapy where a 65 % decrease in SUV was the optimal cutoff to distinguish stage III NSCLC patients with distinct outcome [9]. For stage IV NSCLC, Yoon and colleagues reported that 50 % reduction of SUV could predict time to progression [23]. It is valid to speculate that the optimal cutoff value for favorable subset identification in NSCLC patients receiving definitive chemoradiotherapy should be higher than that in patients with the non-curative or palliative therapy. Accordingly, our study showed that a 75 % of NSUV decrease presented the most discriminative significance in terms of OS and 2-year progression prediction. Although a 60 % reduction was found to be the most robust cutoff for PFS prediction, a 75 % decrease in NSUV still presents adequate value for the identification of better responder.
We acknowledge several limitations in our study. This is a secondary analysis of a series of prospective studies. PET/CT was not required for the follow-up, and thus, no prospectively defined strict timing was used for the posttreatment PET/CT study. In the present study, we did not have the SUV data corrected by lean body mass (SUL) or body surface area (SUVmax-BSA), and therefore, we could not evaluate the response using PERCIST or EORTC methods. However, given the semiquantitative nature and similar cutoff definition, we would expect a strong concordance in response classification between these methods. Further prospective studies are warranted in a larger and independent cohort to validate our findings and hypothesis.
Conclusions
There is a moderate agreement between the visual assessment of PM and semiquantitative inspection of UM criteria for the metabolic response evaluation, with the main discrepancy of the CMR rate. Categorical metabolic response is validated to be strongly predictive of outcome, and visual assessment seems to be sufficient for CMR identification. The numerical percentage reduction of metabolic activity provides an ordinal value that correlates with prolonged survival. At least 75 % decrease of metabolic activity is indicative for a favorable long-term outcome, pending validation by an independent population.
References
Ichiya Y, Kuwabara Y, Sasaki M, Yoshida T, Omagari J, Akashi Y, Kawashima A, Fukumura T, Masuda K (1996) A clinical evaluation of FDG-PET to assess the response in radiation therapy for bronchogenic carcinoma. Ann Nucl Med 10:193–200
Hicks RJ, Kalff V, MacManus MP, Ware RE, McKenzie AF, Matthews JP, Ball DL (2001) The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med 42:1605–1613
Kalff V, Hicks RJ, MacManus MP, Binns DS, McKenzie AF, Ware RE, Hogg A, Ball DL (2001) Clinical impact of (18)F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: a prospective study. J Clin Oncol 19:111–118
Mac Manus MP, Hicks RJ, Ball DL, Kalff V, Matthews JP, Salminen E, Khaw P, Wirth A, Rischin D, McKenzie A (2001) F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with nonsmall cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer 92:886–895
Mac Manus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, Ball DL (2003) Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol 21:1285–1292
Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, Schwaiger M (2003) Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 21:2651–2657
Vansteenkiste J, Fischer BM, Dooms C, Mortensen J (2004) Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol 5:531–540
Pöttgen C, Levegrün S, Theegarten D, Marnitz S, Grehl S, Pink R, Eberhardt W, Stamatis G, Gauler T, Antoch G, Bockisch A, Stuschke M (2006) Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res 12:97–106
Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, Dittmann H, Langen HJ, Bares R (2007) Repeat 18F-FDG PET for monitoring neoadjuvant chemotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 55:165–171
Kong F, Frey K, Quint L, Ten Haken R, Hayman J, Kessler M, Chetty I, Normolle D, Eisbruch A, Lawrence T (2007) A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol 25:3116–3123
de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, Willemsen AT, Pruim J, Corstens FH, Krabbe PF, Oyen WJ (2007) Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med 48:1592–1598
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C, Moreau M, Roelandts M, Alard S, Meert AP, Patz EF, Lafitte JJ, Sculier JP, E. L. C. W. P. f. t. I. L. C. S. Project (2008) Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 3:6–12
Xu X, Yu J, Sun X, Yang G, Li K, Fu Z, Han A, Zheng J (2008) The prognostic value of 18F-fluorodeoxyglucose uptake by using serial positron emission tomography and computed tomography in patients with stage III nonsmall cell lung cancer. Am J Clin Oncol 31:470–475
Allen-Auerbach M, Weber WA (2009) Measuring response with FDG-PET: methodological aspects. Oncologist 14:369–377
van Loon J, Grutters J, Wanders R, Boersma L, Oellers M, Dingemans AM, Bootsma G, Geraedts W, Pitz C, Simons J, Fatah SA, Snoep G, Hochstenbag M, Lambin P, De Ruysscher D (2009) Follow-up with 18FDG-PET-CT after radical radiotherapy with or without chemotherapy allows the detection of potentially curable progressive disease in non-small cell lung cancer patients: a prospective study. Eur J Cancer 45:588–595
Harry VN, Semple SI, Parkin DE, Gilbert FJ (2010) Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol 11:92–102
Lee HY, Lee HJ, Kim YT, Kang CH, Jang BG, Chung DH, Goo JM, Park CM, Lee CH, Kang KW (2010) Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 5:497–503
Obrzut S, Bykowski J, Badran K, Hayeri MR, Hoh CK (2010) Utility of fluorodeoxyglucose-positron emission tomography in the identification of new lesions in lung cancer patients for the assessment of therapy response. Nucl Med Commun 31:1008–1015
Segall GM (2010) Therapy response evaluation with positron emission tomography-computed tomography. Semin Ultrasound CT MR 31:490–495
Shiraishi K, Nomori H, Ohba Y, Kaji M, Mori T, Shibata H, Oya N, Sasaki J (2010) Repeat FDG-PET for predicting pathological tumor response and prognosis after neoadjuvant treatment in nonsmall cell lung cancer: comparison with computed tomography. Ann Thorac Cardiovasc Surg 16:394–400
Afaq A, Akin O (2011) Imaging assessment of tumor response: past, present and future. Future Oncol 7:669–677
Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, Hellmich M, Papachristou I, Toepelt K, Draube A, Heukamp L, Buettner R, Ko YD, Ullrich RT, Smit E, Boellaard R, Lammertsma AA, Hallek M, Jacobs AH, Schlesinger A, Schulte K, Querings S, Stoelben E, Neumaier B, Thomas RK, Dietlein M, Wolf J (2011) Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. J Clin Oncol 29:1701–1708
Yoon DH, Baek S, Choi CM, Lee DH, Suh C, Ryu JS, Moon DH, Lee JS, Kim SW (2011) FDG-PET as a potential tool for selecting patients with advanced non-small cell lung cancer who may be spared maintenance therapy after first-line chemotherapy. Clin Cancer Res 17:5093–5100
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M (1999) Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med 40:1771–1777
Paquet N, Albert A, Foidart J, Hustinx R (2004) Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med 45:784–788
Kuo WH, Wu YC, Wu CY, Ho KC, Chiu PH, Wang CW, Chang CJ, Yu CT, Yen TC, Lin C (2012) Node/aorta and node/liver SUV ratios from (18)F-FDG PET/CT may improve the detection of occult mediastinal lymph node metastases in patients with non-small cell lung carcinoma. Acad Radiol 19:685–692
Skougaard K, Nielsen D, Jensen BV, Hendel HW (2013) Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med 54:1026–1031
Ziai D, Wagner T, El Badaoui A, Hitzel A, Woillard JB, Melloni B, Monteil J (2013) Therapy response evaluation with FDG-PET/CT in small cell lung cancer: a prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imaging 13:73–80
Machtay M, Duan F, Siegel BA, Snyder BS, Gorelick JJ, Reddin JS, Munden R, Johnson DW, Wilf LH, DeNittis A, Sherwin N, Cho KH, Kim SK, Videtic G, Neumann DR, Komaki R, Macapinlac H, Bradley JD, Alavi A (2013) Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol 31:3823–3830
Ethical standard
This is a secondary analysis of a series of prospective studies initiated between 2003 and 2010 at the University of Michigan (UM). All of these studies were approved by the institutional review board (IRB) of the University of Michigan, and written informed consent was obtained from each patient.
Source of funding
This work was funded in part by R01CA142840 to Dr. Feng-Ming (Spring) Kong. This work was presented in part as a poster presentation at the 15th World Conference on Lung Cancer held in Sydney, Australia, October 27–30, 2013.
Conflict of interest
Jingbo Wang, Ka Kit Wong, Morand Piert, Paul Stanton, Kirk A. Frey, and Feng-Ming (Spring) Kong declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Wong, K.K., Piert, M. et al. Metabolic response assessment with 18F-FDG PET/CT: inter-method comparison and prognostic significance for patients with non-small cell lung cancer. J Radiat Oncol 4, 249–256 (2015). https://doi.org/10.1007/s13566-015-0184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-015-0184-9