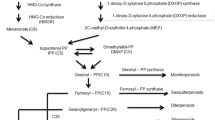
Abstract
We isolated a 2-alkenal reductase (AaAER) gene from Artemisia annua L. cDNA library through homologous cloning strategy. Enzymatic properties of AaAER, including substrate selectivity, enzyme kinetics, and key factors affecting enzyme activity, were characterized in vitro. We found that AaAER mainly functions in catalyzing the reduction of adjacent straight chain “C = C” double bond in the carbonyl group. This suggests the possibility of its participation in downstream events of the HPL (hydroperoxide lyase) pathway and contribution to cell detoxification in plants. Although, AaAER shares some substrates with AaDbr 1 and AaDbr 2, which are involved in artemisinin biosynthesis, AaAER was unable to reduce the adjacent double bond of carbonyl group in artemisinic aldehyde. We also analyzed the possible causes for highly homologous reductases with different types of substrates and non-homologous enzymes sharing the same substrate. The identification of a novel double bond reductase in A. annua provides an opportunity to explore the mechanism underlying the detoxification of aldehyde ketone molecules, which are generated by HPL downstream pathway and the redox process in the metabolic pathway of artemisinin synthesis.


Similar content being viewed by others
Abbreviations
- MeJA:
-
Methyl jasmonate
- SA:
-
Salicylic acid
- RACE:
-
Rapid amplification of cDNA ends
- AaAER:
-
2-alkenal reductase of Artemisia annua
- Dbr1:
-
Double bond reductase 1
- Dbr2:
-
Artemisinic aldehyde Δ11(13) reductase
- MEGA:
-
Molecular evolutionary genetics analysis
References
Chehab EW, Perea JV, Gopalan B, Theg S, Dehesh K (2007) Oxylipin pathway in rice and Arabidopsis. J Integr Plant Biol 49:43–51
Dick RA, Kwak M, Sutter TR, Kensler TW (2001) Antioxidative function and substrate specificity of NAD(P)H dependent alkenal/one oxidoreductase. J Biol Chem 276:40803–40810
Farmer EE, Ryan C (1990) A Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A 87:7713–7716
Hernandez JA, Corpas FJ, Gomez M, del Río LA, Sevilla F (1993) Salt induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89:103–110
Hirata T, Tamura Y, Yokobatake N, Shimoda K, Ashida Y (2000) A 38 kDa allylic alcohol dehydrogenase from the cultured cells of Nicotiana tabacum. Phytochemistry 55:297–303
Mano J, Torii Y, Hayashi S, Takimoto K, Matsui K, Nakamura K, Inze D, Babiychuk E, Kushnir S, Asada K (2002) The NADPH: quinone oxidoreductase P1-ζ-crystallin in Arabidopsis catalyzes the α, β-hydrogenation of 2-alkenals: detoxication of the lipid peroxide-derived reactive aldehydes. Plant Cell Physiol 43:1445–1455
Mano J, Belles-Boix E, Babiychuk E, Inze D, Torii Y, Hiraoka E, Takimoto K, Slooten L, Asada K, Kushnir S (2005) Protection against photooxidative injury of tobacco leaves by 2-alkenal reductase. detoxication of lipid peroxide-derived reactive carbonyls. Plant Physiol 139:1773–1783
Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P (1994) Drought induces oxidative stress in pea plants. Planta 194:346–352
Nordling E, Jornvall H, Persson B (2002) Medium-chain dehydrogenases/reductases (MDR) Family characterizations including genome comparisons and active site modelling. Eur J Biochem 269:4267–4276
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling induced oxidative stress in maize seedlings and regulatory role of hydrogen peroxide. Plant Cell 6:65–74
Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1:404–411
Ringer KL, McConkey ME, Davis EM, Rushing GW, Croteau R (2003) Monoterpene double-bond reductases of the (−)-menthol biosynthetic pathway: isolation and characterization of cDNAs encoding (−)-isopiperitenone reductase and (+)-pulegone reductase of peppermint. Arch Biochem Biophys 418:80–92
Riveros-Rosas H, Julian-Sanchez A, Villalobos-Molina R, Pardo JP, Pina E (2003) Diversity, taxonomy and evolution of medium-chain dehydrogenase/reductase superfamily. Eur J Biochem 270:3309–3334
Strassner J, Schaller F, Frick UB, Howe GA, Weiler EW, Amrhein N, Macheroux P, Schaller A (2002) Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J 32:585–601
Takeuchi Y, Fukumoto R, Kasahara H, Sasaki T, Mitsutoshi K (1995) Peroxidation of lipids and growth inhibition induced by UV-B irradiation. Plant Cell Rep 14:566–570
Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42:318–343
Youn B, Kim S, Moinuddin SGA, Lee C, Bedgar DL, Harper AR, Davin LB, Lewis NG, Kang CH (2006) Mechanistic and structural studies of apoform, binary, and ternary complexes of the Arabidopsis alkenal double bond reductase At5g16970. J Biol Chem 281:40076–40088
Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS (2008) The molecular cloning of artemisinic aldehyde 11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–21508
Zhang Y, Teoh KH, Reed DW, Covello PS (2009) Molecular cloning and characterization of Dbr1, a 2-alkenal reductase from Artemisia annua. Botany 87:643–649
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
The primers used in this study (DOC 32 kb)
Fig. S1
The alignment of amino acid sequences of AaAER and its homologs. The boxed AXXGXXG is NADP + binding site. Three glycines indicated with arrowheads are key sites of the active enzyme with high-level structure. (GIF 189 kb)
Fig. S2
Inducible expression and purification of AaAER. (a) In vitro Inducible expression and purification of AaAER protein. Lane 1, in vitro total AaAER protein; lane 2, empty vector; lane 3, 10 mM imidazol elution; lane 4–5, 20 mM imidazol elution; lane 6–8 250 mM imidazol elution; Lane 9, protein marker. (b) Western blotting data further identified target proteins. Data showed the protein size of the target is around 55 kD. Lane 1, protein target; lane 2, protein marker. (GIF 91 kb)
Fig. S3
GC-MS analyses of the reaction between AaAER and 2-nonenal. Reduction activity of AaAER was identified in reaction systems with inactive proteins and without NADPH. Product was identified through GC-MS chromatogram compared to standard. a, Chromatogram of product (2-nonanal). b, Chromatogram of standard (2-nonanal). (GIF 219 kb)
Fig. S4
GC-MS analyses of the reaction between AaAER and 3-nonen-2-one. Reduction activity of AaAER was identified in reaction systems with inactived proteins. Product was identified through GC-MS chromatogram compared to standard. a, Chromatogram of product (2-nonanone). b, Chromatogram of standard (2-nonanone). (GIF 161 kb)
Fig. S5
GC-MS analyses of the reaction between AaAER and cinnamaldehyde. Reduction activity of AaAER was identified in reaction systems with inactivate proteins. Product was identified through GC-MS chromatogram compared to standard. a, Chromatogram of product (hydrocinnamaldehyde). b, Chromatogram of standard (hydrocinnamaldehyde). (GIF 172 kb)
Fig. S6
GC-MS analyses of the reaction between AaAER and trans-2, cis-6-nonadienal. Product was identified through GC-MS chromatogram compared to standard. a, Chromatogram of product (6-nonenal). b, Chromatogram of standard (6-nonenal). (GIF 198 kb)
Fig. S7
Effect of temperature on AaAER activity. Reactions with 3-nonen-2-one as a substrate were carried out in HEPES buffer at pH 6.0. The curve of reaction rate against the temperature. (GIF 13 kb)
Fig. S8
Effects of pH on AaAER activity. Reactions were carried out in HEPES buffer at 37 °C. The curve of reaction rate against pH. (GIF 12 kb)
Fig. S9
Effects of metal ions on AaAER activity. Comparison of reaction rate with different metal ions. HEPES buffer without metal ions was used as a control. Reactions with 3-nonen-2-one as a substrate were carried out at 37 °C and pH 6.0. The difference in the average reaction rate of different metal ions was evaluated by Dunnett’s Multiple Comparison Test. ** and *** indicate different degrees of difference compared to controls. (GIF 28 kb)
Rights and permissions
About this article
Cite this article
Wei, YK., Li, JX., Hu, WL. et al. Isolation and characterization of AaAER, a novel double bond reductase from Artemisia annua . J. Plant Biochem. Biotechnol. 24, 331–337 (2015). https://doi.org/10.1007/s13562-014-0278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-014-0278-2